Randomized double-blind 2 x 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women
- PMID: 19597031
- PMCID: PMC2799048
- DOI: 10.1200/JCO.2008.19.3797
Randomized double-blind 2 x 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women
Abstract
Purpose: Tamoxifen and fenretinide are active in reducing premenopausal breast cancer risk and work synergistically in preclinical models. The authors assessed their combination in a two-by-two biomarker trial.
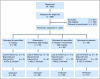
Patients and methods: A total of 235 premenopausal women with pT1mic/pT1a breast cancer (n = 21), or intraepithelial neoplasia (IEN, n = 160), or 5-year Gail risk > or = 1.3% (n = 54) were randomly allocated to either tamoxifen 5 mg/d, fenretinide 200 mg/d, their combination, or placebo. We report data for plasma insulin-like growth factor I (IGF-I), mammographic density, uterine effects, and breast neoplastic events after 5.5 years.
Results: During the 2-year intervention, tamoxifen significantly lowered IGF-I and mammographic density by 12% and 20%, respectively, fenretinide by 4% and 10% (not significantly), their combination by 20% and 22%, with no evidence for a synergistic interaction. Tamoxifen increased endometrial thickness principally in women becoming postmenopausal, whereas fenretinide decreased endometrial thickness significantly. The annual rate of breast neoplasms (n = 48) was 3.5% +/- 1.0%, 2.1% +/- 0.8%, 4.7% +/- 1.3%, and 5.2% +/- 1.3% in the tamoxifen, fenretinide, combination, and placebo arms, respectively, with hazard ratios (HRs) of 0.70 (95% CI, 0.32 to 1.52), 0.38 (95% CI, 0.15 to 0.90), and 0.96 (95% CI, 0.46 to 1.99) relative to placebo (tamoxifen x fenretinide adverse interaction P = .03). There was no clear association with tumor receptor type. Baseline IGF-I and mammographic density did not predict breast neoplastic events, nor did change in mammographic density.
Conclusion: Despite favorable effects on plasma IGF-I levels and mammographic density, the combination of low-dose tamoxifen plus fenretinide did not reduce breast neoplastic events compared to placebo, whereas both single agents, particularly fenretinide, showed numerical reduction in annual odds of breast neoplasms. Further follow-up is indicated.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Surrogate response biomarkers in prevention research: do they point the way or lead us astray?J Clin Oncol. 2009 Aug 10;27(23):3734-6. doi: 10.1200/JCO.2009.22.9211. Epub 2009 Jul 13. J Clin Oncol. 2009. PMID: 19597020 No abstract available.
References
-
- Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91:1829–1846. - PubMed
-
- Veronesi U, Mariani L, Decensi A, et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann Oncol. 2006;17:1065–1071. - PubMed
-
- Ratko TA, Detrisac CJ, Dinger NM, et al. Chemopreventive efficacy of combined retinoid and tamoxifen treatment following surgical excision of a primary mammary cancer in female rats. Cancer Res. 1989;49:4472–4476. - PubMed
-
- Conley B, O'Shaughnessy J, Prindiville S, et al. Pilot trial of the safety, tolerability, and retinoid levels of N-(4-hydroxyphenyl) retinamide in combination with tamoxifen in patients at high risk for developing invasive breast cancer. J Clin Oncol. 2000;18:275–283. - PubMed
-
- Singletary SE, Atkinson EN, Hoque A, et al. Phase II clinical trial of N-(4-Hydroxyphenyl)retinamide and tamoxifen administration before definitive surgery for breast neoplasia. Clin Cancer Res. 2002;8:2835–2842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

