Active adaptation of the tethered mitral valve: insights into a compensatory mechanism for functional mitral regurgitation
- PMID: 19597052
- PMCID: PMC2752046
- DOI: 10.1161/CIRCULATIONAHA.108.846782
Active adaptation of the tethered mitral valve: insights into a compensatory mechanism for functional mitral regurgitation
Abstract
Background: In patients with left ventricular infarction or dilatation, leaflet tethering by displaced papillary muscles frequently induces mitral regurgitation, which doubles mortality. Little is known about the biological potential of the mitral valve (MV) to compensate for ventricular remodeling. We tested the hypothesis that MV leaflet surface area increases over time with mechanical stretch created by papillary muscle displacement through cell activation, not passive stretching.
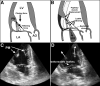
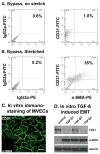
Methods and results: Under cardiopulmonary bypass, the papillary muscle tips in 6 adult sheep were retracted apically short of producing mitral regurgitation to replicate tethering without confounding myocardial infarction or turbulence. Diastolic leaflet area was quantified by 3-dimensional echocardiography over 61+/-6 days compared with 6 unstretched sheep MVs. Total diastolic leaflet area increased by 2.4+/-1.3 cm(2) (17+/-10%) from 14.3+/-1.9 to 16.7+/-1.9 cm(2) (P=0.006) with stretch with no change in the unstretched valves despite sham open heart surgery. Stretched MVs were 2.8 times thicker than normal (1.18+/-0.14 versus 0.42+/-0.14 mm; P<0.0001) at 60 days with an increased spongiosa layer. Endothelial cells (CD31(+)) coexpressing alpha-smooth muscle actin were significantly more common by fluorescent cell sorting in tethered versus normal leaflets (41+/-19% versus 9+/-5%; P=0.02), indicating endothelial-mesenchymal transdifferentiation. alpha-Smooth muscle actin-positive cells appeared in the atrial endothelium, penetrating into the interstitium, with increased collagen deposition. Thickened chordae showed endothelial and subendothelial alpha-smooth muscle actin. Endothelial-mesenchymal transdifferentiation capacity also was demonstrated in cultured MV endothelial cells.
Conclusions: Mechanical stresses imposed by papillary muscle tethering increase MV leaflet area and thickness, with cellular changes suggesting reactivated embryonic developmental pathways. Understanding such actively adaptive mechanisms can potentially provide therapeutic opportunities to augment MV area and reduce ischemic mitral regurgitation.
Conflict of interest statement
Figures






Comment in
-
Mitral leaflet in functional regurgitation: passive bystander or active player?Circulation. 2009 Jul 28;120(4):275-7. doi: 10.1161/CIRCULATIONAHA.109.879957. Epub 2009 Jul 13. Circulation. 2009. PMID: 19597044 No abstract available.
References
-
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. - PubMed
-
- Waller BF, Morrow AG, Maron BJ, Del Negro AA, Kent KM, McGrath FJ, Wallace RB, McIntosh CL, Roberts WC. Etiology of clinically isolated, severe, chronic, pure mitral regurgitation: analysis of 97 patients over 30 years of age having mitral valve replacement. Am Heart J. 1982;104:276–288. - PubMed
-
- Kaul S, Spotnitz WD, Glasheen WP, Touchstone DA. Mechanism of ischemic mitral regurgitation. An experimental evaluation. Circulation. 1991;84:2167–2180. - PubMed
-
- Otsuji Y, Handschumacher MD, Schwammenthal E, Jiang L, Song JK, Guerrero JL, Vlahakes GJ, Levine RA. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation. 1997;96:1999–2008. - PubMed
-
- Sabbah HN, Kono T, Stein PD, Mancini GB, Goldstein S. Left ventricular shape changes during the course of evolving heart failure. Am J Physiol. 1992;263:H266–270. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

