Modified directly observed antiretroviral therapy compared with self-administered therapy in treatment-naive HIV-1-infected patients: a randomized trial
- PMID: 19597072
- PMCID: PMC2771688
- DOI: 10.1001/archinternmed.2009.172
Modified directly observed antiretroviral therapy compared with self-administered therapy in treatment-naive HIV-1-infected patients: a randomized trial
Abstract
Background: Success of antiretroviral therapy depends on high rates of adherence, but few interventions are effective. Our objective was to determine if modified directly observed therapy (mDOT) improves initial antiretroviral success.
Methods: In an open-label, randomized trial comparing mDOT (Monday-Friday for 24 weeks) and self-administered therapy with lopinavir/ritonavir soft gel capsules (800 mg/200 mg), emtricitabine (200 mg), and either extended-release stavudine (100 mg) or tenofovir (300 mg), all taken once daily, 82 participants received mDOT and 161, self-administered therapy. Participant eligibility included a plasma human immunodeficiency virus RNA level higher than 2000 copies/mL and being naïve to antiretroviral therapy. A total of 243 participants were predominantly male (79%) (median age, 38 years), with 84 Latinos (35%), 74 non-Latino blacks (30%), and 79 non-Latino whites (33%). The study was conducted at 23 AIDS Clinical Trials Group (ACTG) sites in the United States and 1 site in South Africa between October 2002 and January 2006. The primary outcome was virologic success at week 24 and secondary outcomes were virologic success, clinical progression, and adherence at week 48.
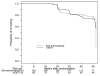
Results: Over 24 weeks, mDOT had greater virologic success (0.91; 95% confidence interval [CI], 0.81 to 0.95) than self-administered therapy (0.84; 95% CI, 0.77 to 0.89), but the difference (0.07; lower bound 95% CI, -0.01) did not reach the prespecified threshold of 0.075. Over 48 weeks, virologic success was not significantly different between mDOT (0.72; 95% CI, 0.61 to 0.81) and self-administered therapy (0.78; 95% CI, 0.70 to 0.84) (difference, -0.06; 95% CI, -0.18 to 0.07 [P = .19]).
Conclusions: The potential benefit of mDOT was marginal and not sustained after discontinuation. Modified DOT should not be incorporated routinely for care of treatment-naïve human immunodeficiency virus type 1-infected patients.
Trial registration: ClinicalTrials.gov NCT00036452.
Figures
Comment in
-
DAART for human immunodeficiency virus-infected patients: Studying subjects not at risk for nonadherence and use of untested interventions.Arch Intern Med. 2010 Jan 11;170(1):109-10. doi: 10.1001/archinternmed.2009.459. Arch Intern Med. 2010. PMID: 20065210 Free PMC article. No abstract available.
References
-
- Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14(4):357–366. - PubMed
-
- Gross R, Bilker WB, Friedman HM, Strom BL. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. AIDS. 2001;15(16):2109–2117. - PubMed
-
- Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. - PubMed
-
- Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006 Aug 9;296(6):679–690. - PubMed
-
- Mills EJ, Nachega JB. A wake-up call for global access to salvage HIV drug regimens. Lancet. 2007 Dec 8;370(9603):1885–1887. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 AI069439/AI/NIAID NIH HHS/United States
- U01 AI069450/AI/NIAID NIH HHS/United States
- U01 AI046381/AI/NIAID NIH HHS/United States
- U01 AI069511/AI/NIAID NIH HHS/United States
- U18 HS016946/HS/AHRQ HHS/United States
- U01 AI069477/AI/NIAID NIH HHS/United States
- U01 AI069513/AI/NIAID NIH HHS/United States
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069501/AI/NIAID NIH HHS/United States
- UM1 AI069472/AI/NIAID NIH HHS/United States
- U01 AI069474/AI/NIAID NIH HHS/United States
- K08 MH01584/MH/NIMH NIH HHS/United States
- U01 AI069434/AI/NIAID NIH HHS/United States
- U01 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069513/AI/NIAID NIH HHS/United States
- M01 RR000096/RR/NCRR NIH HHS/United States
- M01 RR000046/RR/NCRR NIH HHS/United States
- UL1 RR025005/RR/NCRR NIH HHS/United States
- U01AI46370/AI/NIAID NIH HHS/United States
- U01 AI069465/AI/NIAID NIH HHS/United States
- UM1 AI069434/AI/NIAID NIH HHS/United States
- U01 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- U01 AI069428/AI/NIAID NIH HHS/United States
- U01 AI069532/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- M01 RR000044/RR/NCRR NIH HHS/United States
- U01 AI069501/AI/NIAID NIH HHS/United States
- U01 AI68636/AI/NIAID NIH HHS/United States
- UM1 AI069477/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- U01 AI046370/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069474/AI/NIAID NIH HHS/United States
- K08 MH001584/MH/NIMH NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- M01 RR000051/RR/NCRR NIH HHS/United States
- U01 AI025859/AI/NIAID NIH HHS/United States
- UM1 AI069450/AI/NIAID NIH HHS/United States
- UM1 AI069532/AI/NIAID NIH HHS/United States
- UM1 AI069465/AI/NIAID NIH HHS/United States
- UM1 AI069511/AI/NIAID NIH HHS/United States
- U01 AI069494/AI/NIAID NIH HHS/United States
- U01 AI027665/AI/NIAID NIH HHS/United States
- U01 AI032783/AI/NIAID NIH HHS/United States
- U01 AI069472/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States