Phenotypic variability due to a novel Glu292Lys variation in exon 8 of the BEST1 gene causing best macular dystrophy
- PMID: 19597114
- PMCID: PMC2711525
- DOI: 10.1001/archophthalmol.2009.148
Phenotypic variability due to a novel Glu292Lys variation in exon 8 of the BEST1 gene causing best macular dystrophy
Abstract
Objective: To study the phenotypic characteristics of patients with a novel p.E292K mutation in BEST1.
Methods: Affected individuals underwent ophthalmic examination and testing that included photography, autofluorescence, optical coherence tomography, and electrophysiological testing. Their DNA was analyzed for BEST1 mutations.
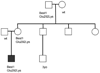
Results: Five patients aged 5 to 59 years who expressed the p.E292K mutation in BEST1 were identified in 3 families. Electro-oculographic light-rise was subnormal in all probands and carriers. Carriers had normal findings from fundus examination, multifocal electroretinography, and visual acuity, and were emmetropic or myopic. Only probands had hyperopia and fundus findings typical of Best macular dystrophy. Optical coherence tomography of vitelliform lesions demonstrated retinal pigment epithelium elevation without subretinal fluid; atrophic lesions exhibited disruption of the hyperreflective outer retina-retinal pigment epithelium complex. Intense hyperautofluorescence correlated with the vitelliform lesion.
Conclusions: Patients with the Glu292Lys variation in BEST1 exhibit intrafamilial and interfamilial phenotypic variability. A disproportionate fraction (26%) of Best disease-causing mutations occurs in exon 8, suggesting that the portion of protein encoded by this exon (amino acids 290-316) may be especially important to bestrophin's function. Relatively good visual acuity with vitelliform lesions can be explained by preservation of the outer retina, demonstrated by optical coherence tomography. Clinical Relevance A novel mutation in this region of BEST1 carries implications for disease pathogenesis.
Figures





Similar articles
-
Phenotypic variability in a French family with a novel mutation in the BEST1 gene causing multifocal best vitelliform macular dystrophy.Mol Vis. 2011 Jan 29;17:309-22. Mol Vis. 2011. PMID: 21293734 Free PMC article.
-
Functional and clinical data of Best vitelliform macular dystrophy patients with mutations in the BEST1 gene.Mol Vis. 2009 Dec 31;15:2960-72. Mol Vis. 2009. PMID: 20057903 Free PMC article.
-
A NOVEL P.ASP304GLY MUTATION IN BEST1 GENE ASSOCIATED WITH ATYPICAL BEST VITELLIFORM MACULAR DYSTROPHY PHENOTYPE AND HIGH INTRAFAMILIAL VARIABILITY.Retina. 2016 Sep;36(9):1733-40. doi: 10.1097/IAE.0000000000000966. Retina. 2016. PMID: 26807628
-
Bestrophins and retinopathies.Pflugers Arch. 2010 Jul;460(2):559-69. doi: 10.1007/s00424-010-0821-5. Epub 2010 Mar 28. Pflugers Arch. 2010. PMID: 20349192 Free PMC article. Review.
-
Functional roles of bestrophins in ocular epithelia.Prog Retin Eye Res. 2009 May;28(3):206-26. doi: 10.1016/j.preteyeres.2009.04.004. Epub 2009 May 4. Prog Retin Eye Res. 2009. PMID: 19398034 Free PMC article. Review.
Cited by
-
Screening for BEST1 gene mutations in Chinese patients with bestrophinopathy.Mol Vis. 2014 Nov 11;20:1594-604. eCollection 2014. Mol Vis. 2014. PMID: 25489231 Free PMC article.
-
Autosomal recessive bestrophinopathy associated with angle-closure glaucoma.Doc Ophthalmol. 2014 Aug;129(1):57-63. doi: 10.1007/s10633-014-9444-z. Epub 2014 May 24. Doc Ophthalmol. 2014. PMID: 24859690 Free PMC article.
-
Best Disease: Global Mutations Review, Genotype-Phenotype Correlation, and Prevalence Analysis in the Israeli Population.Invest Ophthalmol Vis Sci. 2024 Feb 1;65(2):39. doi: 10.1167/iovs.65.2.39. Invest Ophthalmol Vis Sci. 2024. PMID: 38411968 Free PMC article.
-
Pharmacological Modulation of Photoreceptor Outer Segment Degradation in a Human iPS Cell Model of Inherited Macular Degeneration.Mol Ther. 2015 Nov;23(11):1700-1711. doi: 10.1038/mt.2015.141. Epub 2015 Aug 24. Mol Ther. 2015. PMID: 26300224 Free PMC article.
-
Choroidal Neovascularization Is Common in Best Vitelliform Macular Dystrophy and Plays a Role in Vitelliform Lesion Evolution.Ophthalmol Retina. 2023 May;7(5):441-449. doi: 10.1016/j.oret.2022.11.014. Epub 2022 Dec 14. Ophthalmol Retina. 2023. PMID: 36528270 Free PMC article.
References
-
- Petrukhin K, Koisti MJ, Bakall B, et al. Identification of the gene responsible for Best macular dystrophy. Nat Genet. 1998;19(3):241–247. - PubMed
-
- Marquardt A, Stöhr H, Passmore LA, Krämer F, Rivera A, Weber BH. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best's disease) Hum Mol Genet. 1998;7(9):1517–1525. - PubMed
-
- Marmorstein AD, Marmorstein LY, Rayborn M, Wang X, Hollyfield JG, Petrukhin K. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2000;97(23):12758–12763. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

