Distinct opioid circuits determine the palatability and the desirability of rewarding events
- PMID: 19597155
- PMCID: PMC2718390
- DOI: 10.1073/pnas.0905874106
Distinct opioid circuits determine the palatability and the desirability of rewarding events
Abstract
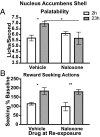
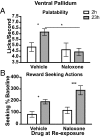
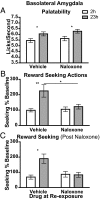
It generally is assumed that a common neural substrate mediates both the palatability and the reward value of nutritive events. However, recent evidence suggests this assumption may not be true. Whereas opioid circuitry in both the nucleus accumbens and ventral pallidum has been reported to mediate taste-reactivity responses to palatable events, the assignment of reward or inventive value to goal-directed actions has been found to involve the basolateral amygdala. Here we found that, in rats, the neural processes mediating palatability and incentive value are indeed dissociable. Naloxone infused into either the ventral pallidum or nucleus accumbens shell blocked the increase in sucrose palatability induced by an increase in food deprivation without affecting the performance of sucrose-related actions. Conversely, naloxone infused into the basolateral amygdala blocked food deprivation-induced changes in sucrose-related actions without affecting sucrose palatability. This double dissociation of opioid-mediated changes in palatability and incentive value suggests that the role of endogenous opioids in reward processing does not depend on a single neural circuit. Rather, changes in palatability and in the incentive value assigned to rewarding events seem to be mediated by distinct neural processes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Balleine BW, Dickinson A. Goal-directed instrumental action: Contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37(4–5):407–419. - PubMed
-
- Cabanac M. Pleasure: The common currency. J Theor Biol. 1992;155(2):173–200. - PubMed
-
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1413–1420. - PubMed
-
- Panksepp J. Affective consciousness: Core emotional feelings in animals and humans. Consciousness and Cognition. 2005;14(1):30–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

