Quantification of nano-scale intermembrane contact areas by using fluorescence resonance energy transfer
- PMID: 19597158
- PMCID: PMC2709668
- DOI: 10.1073/pnas.0903052106
Quantification of nano-scale intermembrane contact areas by using fluorescence resonance energy transfer
Abstract
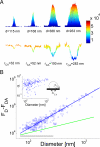
Nanometer-scale intermembrane contact areas (CAs) formed between single small unilamellar lipid vesicles (SUVs) and planar supported lipid bilayers are quantified by measuring fluorescence resonance energy transfer (FRET) between a homogenous layer of donor fluorophores labeling the supported bilayer and acceptor fluorophores labeling the SUVs. The smallest CAs detected in our setup between biotinylated SUVs and dense monolayers of streptavidin were approximately 20 nm in radius. Deformation of SUVs is revealed by comparing the quenching of the donors to calculations of FRET between a perfectly spherical shell and a flat surface containing complementary fluorophores. These results confirmed the theoretical prediction that the degree of deformation scales with the SUV diameter. The size of the CA can be controlled experimentally by conjugating polyethylene glycol polymers to the SUV or the surface and thereby modulating the interfacial energy of adhesion. In this manner, we could achieve secure immobilization of SUVs under conditions of minimal deformation. Finally, we demonstrate that kinetic measurements of CA, at constant adhesion, can be used to record in real-time quantitative changes in the bilayer tension of a nano-scale lipid membrane system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Monks CRF, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T-cells. Nature. 1998;395:82–86. - PubMed
-
- Dustin ML, Colman DR. Neural and immunological synaptic relations. Science. 2002;298:785–789. - PubMed
-
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6:57–69. - PubMed
-
- Zenisek D, Steyer JA, Almers W. Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature. 2000;406:849–854. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

