Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling
- PMID: 19597499
- PMCID: PMC2757056
- DOI: 10.1038/ni.1766
Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling
Abstract
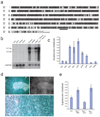
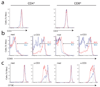
Themis (thymocyte-expressed molecule involved in selection), a member of a family of proteins with unknown functions, is highly conserved among vertebrates. Here we found that Themis had high expression in thymocytes between the pre-T cell antigen receptor (pre-TCR) and positive-selection checkpoints and low expression in mature T cells. Themis-deficient thymocytes showed defective positive selection, which resulted in fewer mature thymocytes. Negative selection was also impaired in Themis-deficient mice. A greater percentage of Themis-deficient T cells had CD4(+)CD25(+)Foxp3(+) regulatory and CD62L(lo)CD44(hi) memory phenotypes than did wild-type T cells. In support of the idea that Themis is involved in TCR signaling, this protein was phosphorylated quickly after TCR stimulation and was needed for optimal TCR-driven calcium mobilization and activation of the kinase Erk.
Conflict of interest statement
The authors have no conflicting financial interests.
Figures

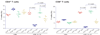



Comment in
-
Themis imposes new law and order on positive selection.Nat Immunol. 2009 Aug;10(8):805-6. doi: 10.1038/ni0809-805. Nat Immunol. 2009. PMID: 19621038 No abstract available.
References
-
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu. Rev. Immunol. 2003;21:139–176. - PubMed
-
- Werlen G, Hausmann B, Naeher D, Palmer E. Signaling life and death in the thymus: timing is everything. Science. 2003;299:1859–1863. - PubMed
-
- Malissen B, Malissen M. Allelic exclusion of T cell antigen receptor genes. In: Bell JI, Owen MJ, Simpson E, editors. T cell receptors. Oxford: Oxford University Press; 1995. pp. 352–368.
-
- Lucas B, Stefanova I, Yasutomo K, Dautigny N, Germain RN. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 1999;10:367–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

