Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity
- PMID: 19597562
- PMCID: PMC2708966
- DOI: 10.3389/neuro.04.005.2009
Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity
Abstract
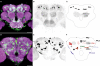
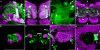
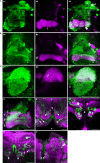
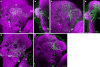
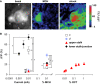
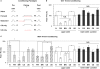
We examined tyrosine hydroxylase (TH-GAL4) expression and anti-TH immunoreactivity in the Drosophila protocerebrum and characterized single cell clones of the TH-GAL4 neurons. Eight clusters of putative dopaminergic neurons were characterized. Neurons in three of the clusters project to the mushroom body neuropil: PAM neurons project to the medial portion of the horizontal lobes; PPL1 neurons project to the vertical lobes, the junction area, the heel and distal peduncle; and PPL2ab neurons project to the calyx. Five types of PPL1 neurons were discovered that innervate different zones of the mushroom body lobes. Functional imaging experiments showed that the dopaminergic processes in four of the zones differ in response properties to odor, electric shock, or following the pairing of odor and electric shock. These results indicate that distinct dopaminergic neurons define separate zones of the mushroom body lobes and are probably involved in different functions. Differences in functional response properties of these neurons suggest that they are involved in different behavioral processes.
Keywords: Drosophila; dopamine; functional optical imaging; olfactory learning; single cell clones; tyrosine hydroxylase.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

