Inactivation of fibroblast growth factor receptor signaling in myelinating glial cells results in significant loss of adult spiral ganglion neurons accompanied by age-related hearing impairment
- PMID: 19598249
- PMCID: PMC2900924
- DOI: 10.1002/jnr.22164
Inactivation of fibroblast growth factor receptor signaling in myelinating glial cells results in significant loss of adult spiral ganglion neurons accompanied by age-related hearing impairment
Abstract
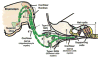
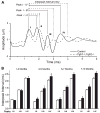
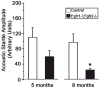
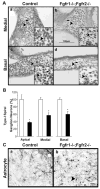
Hearing loss has been attributed to many factors, including degeneration of sensory neurons in the auditory pathway and demyelination along the cochlear nerve. Fibroblast growth factors (FGFs), which signal through four receptors (Fgfrs), are produced by auditory neurons and play a key role in embryonic development of the cochlea and in neuroprotection against sound-induced injury. However, the role of FGF signaling in the maintenance of normal auditory function in adult and aging mice remains to be elucidated. Furthermore, the contribution of glial cells, which myelinate the cochlear nerves, is poorly understood. To address these questions, we generated transgenic mice in which Fgfr1 and Fgfr2 were specifically inactivated in Schwann cells and oligodendrocytes but not in neurons. Adult mutant mice exhibited late onset of hearing impairment, which progressed markedly with age. The hearing impairment was accompanied by significant loss of myelinated spiral ganglion neurons. The pathology extended into the cochlear nucleus, without apparent loss of myelin or of the deletion-bearing glial cells themselves. This suggests that perturbation of FGF receptor-mediated glial function leads to the attenuation of glial support of neurons, leading to their loss and impairment of auditory functions. Thus, FGF/FGF receptor signaling provides a potentially novel mechanism of maintaining reciprocal interactions between neurons and glia in adult and aging animals. Dysfunction of glial cells and FGF receptor signaling may therefore be implicated in neurodegenerative hearing loss associated with normal aging.
Figures



Similar articles
-
Noise-Induced Dysregulation of Quaking RNA Binding Proteins Contributes to Auditory Nerve Demyelination and Hearing Loss.J Neurosci. 2018 Mar 7;38(10):2551-2568. doi: 10.1523/JNEUROSCI.2487-17.2018. Epub 2018 Feb 6. J Neurosci. 2018. PMID: 29437856 Free PMC article.
-
Disruption of fibroblast growth factor receptor signaling in nonmyelinating Schwann cells causes sensory axonal neuropathy and impairment of thermal pain sensitivity.J Neurosci. 2009 Feb 11;29(6):1608-14. doi: 10.1523/JNEUROSCI.5615-08.2009. J Neurosci. 2009. PMID: 19211868 Free PMC article.
-
FGF Signaling Is Necessary for Neurogenesis in Young Mice and Sufficient to Reverse Its Decline in Old Mice.J Neurosci. 2015 Jul 15;35(28):10217-23. doi: 10.1523/JNEUROSCI.1469-15.2015. J Neurosci. 2015. PMID: 26180198 Free PMC article.
-
Axon-glial signaling and the glial support of axon function.Annu Rev Neurosci. 2008;31:535-61. doi: 10.1146/annurev.neuro.30.051606.094309. Annu Rev Neurosci. 2008. PMID: 18558866 Review.
-
Age-related loss of spiral ganglion neurons.Hear Res. 2010 Jun 1;264(1-2):93-7. doi: 10.1016/j.heares.2009.10.009. Epub 2009 Oct 23. Hear Res. 2010. PMID: 19854255 Free PMC article. Review.
Cited by
-
Age-related changes of myelin basic protein in mouse and human auditory nerve.PLoS One. 2012;7(4):e34500. doi: 10.1371/journal.pone.0034500. Epub 2012 Apr 5. PLoS One. 2012. PMID: 22496821 Free PMC article.
-
A spatial and temporal gradient of Fgf differentially regulates distinct stages of neural development in the zebrafish inner ear.PLoS Genet. 2012;8(11):e1003068. doi: 10.1371/journal.pgen.1003068. Epub 2012 Nov 15. PLoS Genet. 2012. PMID: 23166517 Free PMC article.
-
Fibroblast growth factor receptor signaling in oligodendrocytes regulates myelin sheath thickness.J Neurosci. 2012 May 9;32(19):6631-41. doi: 10.1523/JNEUROSCI.6005-11.2012. J Neurosci. 2012. PMID: 22573685 Free PMC article.
-
Expression patterns of FGF receptors in the developing mammalian cochlea.Dev Dyn. 2010 Mar;239(3):1019-26. doi: 10.1002/dvdy.22236. Dev Dyn. 2010. PMID: 20131355 Free PMC article.
-
Role of ERK1/2 MAPK signaling in the maintenance of myelin and axonal integrity in the adult CNS.J Neurosci. 2014 Nov 26;34(48):16031-45. doi: 10.1523/JNEUROSCI.3360-14.2014. J Neurosci. 2014. PMID: 25429144 Free PMC article.
References
-
- Adams JC, Schulte BA. Histopathologic observations of the aging gerbil cochlea. Hear Res. 1997;104:101–111. - PubMed
-
- Bansal R. Fibroblast growth factors and their receptors in oligodendrocyte development: implications for demyelination and remyelination. Dev Neurosci. 2002;24:35–46. - PubMed
-
- Bansal R, Kumar M, Murray K, Morrison RS, Pfeiffer SE. Regulation of FGF receptors in the oligodendrocyte lineage. Mol Cell Neurosci. 1996;7:263–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

