Hydrogels cross-linked by native chemical ligation
- PMID: 19601644
- PMCID: PMC2790863
- DOI: 10.1021/bm900366e
Hydrogels cross-linked by native chemical ligation
Abstract
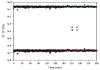
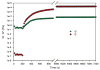
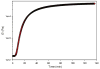
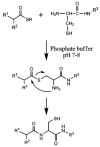
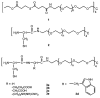
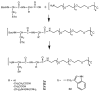
We describe the use of native chemical ligation (NCL) reaction to covalently cross-link soluble polymers into hydrogels. Macromonomers consisting of a four-armed poly(ethylene glycol) (PEG) core end-functionalized with either thioester or N-terminal cysteine peptide were designed and synthesized. Upon mixing aqueous solutions of the thioester and N-terminal cysteine macromonomers, rigid hydrogels formed within minutes. The gelation time was affected by choice of buffer, pH, polymer concentration, reaction temperature, and chemical composition of the N-terminal cysteine conjugate. The kinetics of gel formation and the viscoelastic behavior of selected hydrogels were further studied by oscillatory rheology, which demonstrated a minimum gel formation time of approximately two minutes and the formation of an elastic cross-linked hydrogel via the NCL reaction. A useful feature of this hydrogel strategy is the regeneration of thiol functional groups as a result of the NCL reaction, thereby allowing functionalization of the polymer hydrogel with biomolecules. This was demonstrated by conjugation of a maleimide-GRGDSPG-NH(2) peptide to an NCL hydrogel, permitting the attachment of human mesenchymal stem cells (hMSCs) on the hydrogel. Due to the mild reaction conditions, chemoselectivity, and potential for biological functionalization, our approach may prove useful as a general method for hydrogel formation, including hydrogels intended for biomedical applications.
Figures


References
-
- Hoffman AS. Hydrogels for biomedical applications. Adv Drug Delivery Rev. 2002;54(1):3–12. - PubMed
-
- Peppas NA. Hydrogels in Medicine and Pharmacy. CRC Press; Boca Raton, FL: 1987.
-
- Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18(11):1345–1360.
-
- Okano T. Biorelated Polymers and Gels. Academic Press; Boston: 1998.
-
- Kumar MNVR, Kumar N, Domb AJ, Arora M. Pharmaceutical polymeric controlled drug delivery systems. Adv Polym Sci. 2002;160:45–117.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

