The influence of repair pathways on the cytotoxicity and mutagenicity induced by the pyridyloxobutylation pathway of tobacco-specific nitrosamines
- PMID: 19601657
- PMCID: PMC2787827
- DOI: 10.1021/tx9001572
The influence of repair pathways on the cytotoxicity and mutagenicity induced by the pyridyloxobutylation pathway of tobacco-specific nitrosamines
Abstract
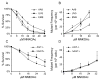
Tobacco-specific nitrosamines, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N'-nitrosonornicotine, are considered to be human carcinogens. Both compounds are metabolized to pyridyloxobutylating intermediates that react with DNA to form adducts such as 7-[4-(3-pyridyl)-4-oxobut-1-yl]guanine, O(2)-[4-(3-pyridyl)-4-oxobut-1-yl]cytosine, O(2)-[4-(3-pyridyl)-4-oxobut-1-yl]-2'-deoxythymidine (O(2)-pobdT), O(6)-[4-(3-pyridyl)-4-oxobut-1-yl]-2'-deoxyguanosine (O(6)-pobdG), and 4-hydroxy-1-(3-pyridyl)-1-butanone-releasing adducts. The role of specific DNA adducts in the overall genotoxic activity of the pyridyloxobutylation pathway is not known. One adduct, O(6)-pobdG, is mutagenic. To characterize the mutagenic and cytotoxic properties of pyridyloxobutyl DNA adducts, the impact of DNA repair pathways on the cytotoxic and mutagenic properties of the model pyridyloxobutylating agent, 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone (NNKOAc), was investigated in Chinese hamster ovary cell lines proficient or deficient in O(6)-alkylguanine DNA alkyltransferase (AGT), deficient in both AGT and base excision repair (BER), or deficient in both AGT and nucleotide excision repair (NER). The repair of the four pyridyloxobutyl DNA adducts was determined in the same cell lines via sensitive LC-MS/MS methods. NNKOAc was more cytotoxic in the cell lines lacking AGT, BER, and NER repair pathways. It also induced more mutations in the hprt gene in the BER- and NER-deficient cell lines. However, AGT expression did not influence NNKOAc's mutagenicity despite efficient repair of O(6)-pobdG. Analysis of the hprt mutational spectra indicated that NNKOAc primarily caused point mutations at AT base pairs. GC to AT transition mutations were a minor contributor to the overall mutation spectrum, providing a rationale for the observation that AGT does not protect against the overall mutagenic properties of NNKOAc in this model system. The only adduct affected by the absence of effective NER was O(2)-pobdT. Slower repair of O(2)-pobdT in NER-deficient cells was associated with increased AT to TA transversion mutations, supporting the hypothesis that these mutations are caused by O(2)-pobdT. Together, these data support a hypothesis that the pyridyloxobutylation pathway generates multiple mutagenic and toxic adducts.
Figures
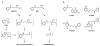
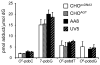
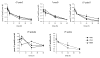
References
-
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:560–603. - PubMed
-
- Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Instit. 1999;91:1194–1210. - PubMed
-
- Hecht SS, Spratt TE, Trushin N. Evidence for 4-(3-pyridyl)-4-oxobutylation of DNA in F344 rats treated with the tobacco specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N′-nitrosonornicotine. Carcinogenesis. 1988;9:161–165. - PubMed
-
- Peterson LA, Hecht SS. O6-Methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung. Cancer Res. 1991;51:5557–5564. - PubMed
-
- Wang M, Cheng G, Sturla SJ, McIntee EJ, Villalta PW, Upadhyaya P, Hecht SS. Identification of adducts formed by pyridyloxobutylation of deoxyguanosine and DNA by 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone, a chemically activated form of tobacco specific carcinogens. Chem Res Toxicol. 2003;16:616–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

