Yersinia outer protein YopE affects the actin cytoskeleton in Dictyostelium discoideum through targeting of multiple Rho family GTPases
- PMID: 19602247
- PMCID: PMC2724381
- DOI: 10.1186/1471-2180-9-138
Yersinia outer protein YopE affects the actin cytoskeleton in Dictyostelium discoideum through targeting of multiple Rho family GTPases
Abstract
Background: All human pathogenic Yersinia species share a virulence-associated type III secretion system that translocates Yersinia effector proteins into host cells to counteract infection-induced signaling responses and prevent phagocytosis. Dictyostelium discoideum has been recently used to study the effects of bacterial virulence factors produced by internalized pathogens. In this study we explored the potential of Dictyostelium as model organism for analyzing the effects of ectopically expressed Yersinia outer proteins (Yops).
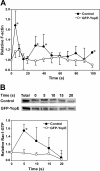
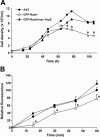
Results: The Yersinia pseudotuberculosis virulence factors YopE, YopH, YopM and YopJ were expressed de novo within Dictyostelium and their effects on growth in axenic medium and on bacterial lawns were analyzed. No severe effect was observed for YopH, YopJ and YopM, but expression of YopE, which is a GTPase activating protein for Rho GTPases, was found to be highly detrimental. GFP-tagged YopE expressing cells had less conspicuous cortical actin accumulation and decreased amounts of F-actin. The actin polymerization response upon cAMP stimulation was impaired, although chemotaxis was unaffected. YopE also caused reduced uptake of yeast particles. These alterations are probably due to impaired Rac1 activation. We also found that YopE predominantly associates with intracellular membranes including the Golgi apparatus and inhibits the function of moderately overexpressed RacH.
Conclusion: The phenotype elicited by YopE in Dictyostelium can be explained, at least in part, by inactivation of one or more Rho family GTPases. It further demonstrates that the social amoeba Dictyostelium discoideum can be used as an efficient and easy-to-handle model organism in order to analyze the function of a translocated GAP protein of a human pathogen.
Figures

Similar articles
-
YopE of Yersinia, a GAP for Rho GTPases, selectively modulates Rac-dependent actin structures in endothelial cells.Cell Microbiol. 2001 May;3(5):301-10. doi: 10.1046/j.1462-5822.2001.00114.x. Cell Microbiol. 2001. PMID: 11298653
-
Modulation of Rho GTPases and the actin cytoskeleton by Yersinia outer proteins (Yops).Int J Med Microbiol. 2001 Sep;291(4):269-76. doi: 10.1078/1438-4221-00130. Int J Med Microbiol. 2001. PMID: 11680787 Review.
-
GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure.Mol Microbiol. 2000 May;36(3):737-48. doi: 10.1046/j.1365-2958.2000.01898.x. Mol Microbiol. 2000. PMID: 10844661
-
The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence.Mol Microbiol. 2000 Aug;37(3):515-27. doi: 10.1046/j.1365-2958.2000.02021.x. Mol Microbiol. 2000. PMID: 10931345
-
Cellular mechanisms of bacterial internalization counteracted by Yersinia.Int Rev Cytol. 2005;246:135-88. doi: 10.1016/S0074-7696(05)46004-0. Int Rev Cytol. 2005. PMID: 16164968 Review.
Cited by
-
The Yersinia pestis Effector YopM Inhibits Pyrin Inflammasome Activation.PLoS Pathog. 2016 Dec 2;12(12):e1006035. doi: 10.1371/journal.ppat.1006035. eCollection 2016 Dec. PLoS Pathog. 2016. PMID: 27911947 Free PMC article.
-
VopX, a novel Vibrio cholerae T3SS effector, modulates host actin dynamics.mBio. 2025 Mar 12;16(3):e0301824. doi: 10.1128/mbio.03018-24. Epub 2025 Jan 29. mBio. 2025. PMID: 39878476 Free PMC article.
-
Mycobacterium marinum mmar_2318 and mmar_2319 are Responsible for Lipooligosaccharide Biosynthesis and Virulence Toward Dictyostelium.Front Microbiol. 2016 Jan 7;6:1458. doi: 10.3389/fmicb.2015.01458. eCollection 2015. Front Microbiol. 2016. PMID: 26779131 Free PMC article.
-
Use of a Dictyostelium model for isolation of genetic loci associated with phagocytosis and virulence in Klebsiella pneumoniae.Infect Immun. 2011 Mar;79(3):997-1006. doi: 10.1128/IAI.00906-10. Epub 2010 Dec 20. Infect Immun. 2011. PMID: 21173313 Free PMC article.
-
Amoebae as Targets for Toxins or Effectors Secreted by Mammalian Pathogens.Toxins (Basel). 2021 Jul 28;13(8):526. doi: 10.3390/toxins13080526. Toxins (Basel). 2021. PMID: 34437397 Free PMC article. Review.
References
-
- DeLeo FR, Hinnebusch BJ. A plague upon the phagocytes. Nat Med. 2005;11:927–928. - PubMed
-
- Cornelis GR. How Yops find their way out of Yersinia. Mol Microbiol. 2003;50:1091–1094. - PubMed
-
- Aepfelbacher M, Trasak C, Ruckdeschel K. Effector functions of pathogenic Yersinia species. Thromb Haemost. 2007;98:521–529. - PubMed
-
- Deleuil F, Mogemark L, Francis MS, Wolf-Watz H, Fallman M. Interaction between the Yersinia protein tyrosine phosphatase YopH and eukaryotic Cas/Fyb is an important virulence mechanism. Cell Microbiol. 2003;5:53–64. - PubMed
-
- Bruckner S, Rhamouni S, Tautz L, Denault JB, Alonso A, Becattini B, Salvesen GS, Mustelin T. Yersinia phosphatase induces mitochondrially dependent apoptosis of T cells. J Biol Chem. 2005;280:10388–10394. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

