Generation of novel AAV variants by directed evolution for improved CFTR delivery to human ciliated airway epithelium
- PMID: 19603002
- PMCID: PMC2801879
- DOI: 10.1038/mt.2009.155
Generation of novel AAV variants by directed evolution for improved CFTR delivery to human ciliated airway epithelium
Abstract
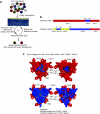
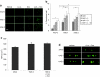
Recombinant adeno-associated virus (AAV) vectors expressing the cystic fibrosis transmembrane conductance regulator (CFTR) gene have been used to deliver CFTR to the airway epithelium of cystic fibrosis (CF) patients. However, no significant CFTR function has been demonstrated likely due to low transduction efficiencies of the AAV vectors. To improve AAV transduction efficiency for human airway epithelium (HAE), we generated a chimeric AAV library and performed directed evolution of AAV on an in vitro model of human ciliated airway epithelium. Two independent and novel AAV variants were identified that contained capsid components from AAV-1, AAV-6, and/or AAV-9. The transduction efficiencies of the two novel AAV variants for human ciliated airway epithelium were three times higher than that for AAV-6. The novel variants were then used to deliver CFTR to ciliated airway epithelium from CF patients. Here we show that our novel AAV variants, but not the parental, AAV provide sufficient CFTR delivery to correct the chloride ion transport defect to ~25% levels measured in non-CF cells. These results suggest that directed evolution of AAV on relevant in vitro models will enable further improvements in CFTR gene transfer efficiency and the development of an efficacious and safe gene transfer vector for CF lung disease.
Figures



References
-
- Koch C., and , Høiby N. Pathogenesis of cystic fibrosis. Lancet. 1993;341:1065–1069. - PubMed
-
- Moss RB, Milla C, Colombo J, Accurso F, Zeitlin PL, Clancy JP, et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum Gene Ther. 2007;18:726–732. - PubMed
-
- Flotte TR. Recent developments in recombinant AAV-mediated gene therapy for lung diseases. Curr Gene Ther. 2005;5:361–366. - PubMed
-
- Duan D, Yue Y, Yan Z., and , Engelhardt JF. A new dual-vector approach to enhance recombinant adeno-associated virus-mediated gene expression through intermolecular cis activation. Nat Med. 2000;6:595–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

