KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer
- PMID: 19603018
- PMCID: PMC2736831
- DOI: 10.1038/sj.bjc.6605177
KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer
Abstract
Background: KRAS codons 12 and 13 mutations predict resistance to anti-EGFR monoclonal antibodies (moAbs) in metastatic colorectal cancer. Also, BRAF V600E mutation has been associated with resistance. Additional KRAS mutations are described in CRC.
Methods: We investigated the role of KRAS codons 61 and 146 and BRAF V600E mutations in predicting resistance to cetuximab plus irinotecan in a cohort of KRAS codons 12 and 13 wild-type patients.
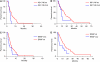
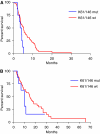
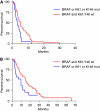
Results: Among 87 KRAS codons 12 and 13 wild-type patients, KRAS codons 61 and 146 were mutated in 7 and 1 case, respectively. None of mutated patients responded vs 22 of 68 wild type (P=0.096). Eleven patients were not evaluable. KRAS mutations were associated with shorter progression-free survival (PFS, HR: 0.46, P=0.028). None of 13 BRAF-mutated patients responded vs 24 of 74 BRAF wild type (P=0.016). BRAF mutation was associated with a trend towards shorter PFS (HR: 0.59, P=0.073). In the subgroup of BRAF wild-type patients, KRAS codons 61/146 mutations determined a lower response rate (0 vs 37%, P=0.047) and worse PFS (HR: 0.45, P=0.023). Patients bearing KRAS or BRAF mutations had poorer response rate (0 vs 37%, P=0.0005) and PFS (HR: 0.51, P=0.006) compared with KRAS and BRAF wild-type patients.
Conclusion: Assessing KRAS codons 61/146 and BRAF V600E mutations might help optimising the selection of the candidate patients to receive anti-EGFR moAbs.
Figures

Comment in
-
KRAS status analysis and anti-EGFR therapies: is comprehensiveness a biologist's fancy or a clinical necessity?Br J Cancer. 2010 Mar 16;102(6):1074-5; author reply 1076-7. doi: 10.1038/sj.bjc.6605582. Epub 2010 Feb 16. Br J Cancer. 2010. PMID: 20160721 Free PMC article. No abstract available.
References
-
- Adam R, Wicherts DA, de Haas RJ, Ciacio O, Levi F, Paule B, Ducreux M, Azoulay D, Bismuth H, Castaing D (2009) Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 27(11): 1829–1835 - PubMed
-
- Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, McAllister PK, Morton RF, Schilsky RL (2009) American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 27(12): 2091–2096 - PubMed
-
- Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26(10): 1626–1634 - PubMed
-
- Bos JL (1989) Ras oncogenes in human cancer: a review. Cancer Res 49(17): 4682–4689 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

