Identification of flavopiridol analogues that selectively inhibit positive transcription elongation factor (P-TEFb) and block HIV-1 replication
- PMID: 19603446
- PMCID: PMC2754223
- DOI: 10.1002/cbic.200900303
Identification of flavopiridol analogues that selectively inhibit positive transcription elongation factor (P-TEFb) and block HIV-1 replication
Abstract
The positive transcription elongation factor (P-TEFb; CDK9/cyclin T1) regulates RNA polymerase II-dependent transcription of cellular and integrated viral genes. It is an essential cofactor for HIV-1 Tat transactivation, and selective inhibition of P-TEFb blocks HIV-1 replication without affecting cellular transcription; this indicates that P-TEFb could be a potential target for developing anti-HIV-1 therapeutics. Flavopiridol, a small molecule CDK inhibitor, blocks HIV-1 Tat transactivation and viral replication by inhibiting P-TEFb kinase activity, but it is highly cytotoxic. In the search for selective and less cytotoxic P-TEFb inhibitors, we prepared a series of flavopiridol analogues and evaluated their kinase inhibitory activity against P-TEFb and CDK2/cyclin A, and tested their cellular antiviral potency and cytotoxicity. We identified several analogues that selectively inhibit P-TEFb kinase activity in vitro and show antiviral potency comparable to that of flavopiridol, but with significantly reduced cytotoxicity. These compounds are valuable molecular probes for understanding P-TEFb-regulated cellular and HIV-1 gene transcription and provide potential anti-HIV-1 therapeutics.
Figures
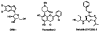
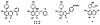

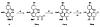
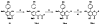
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

