Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice
- PMID: 19603549
- PMCID: PMC2701862
- DOI: 10.1172/jci37059
Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice
Abstract
A trial fibrillation (AF), the most common human cardiac arrhythmia, is associated with abnormal intracellular Ca2+ handling. Diastolic Ca2+ release from the sarcoplasmic reticulum via "leaky" ryanodine receptors (RyR2s) is hypothesized to contribute to arrhythmogenesis in AF, but the molecular mechanisms are incompletely understood. Here, we have shown that mice with a genetic gain-of-function defect in Ryr2 (which we termed Ryr2R176Q/+ mice) did not exhibit spontaneous AF but that rapid atrial pacing unmasked an increased vulnerability to AF in these mice compared with wild-type mice. Rapid atrial pacing resulted in increased Ca2+/calmodulin-dependent protein kinase II (CaMKII) phosphorylation of RyR2, while both pharmacologic and genetic inhibition of CaMKII prevented AF inducibility in Ryr2R176Q/+ mice. This result suggests that AF requires both an arrhythmogenic substrate (e.g., RyR2 mutation) and enhanced CaMKII activity. Increased CaMKII phosphorylation of RyR2 was observed in atrial biopsies from mice with atrial enlargement and spontaneous AF, goats with lone AF, and patients with chronic AF. Genetic inhibition of CaMKII phosphorylation of RyR2 in Ryr2S2814A knockin mice reduced AF inducibility in a vagotonic AF model. Together, these findings suggest that increased RyR2-dependent Ca2+ leakage due to enhanced CaMKII activity is an important downstream effect of CaMKII in individuals susceptible to AF induction.
Figures
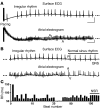
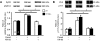





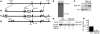
References
-
- Van Wagoner D.R., et al. Atrial L-type Ca2+ currents and human atrial fibrillation. . Circ. Res. 1999;85:428–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL062494/HL/NHLBI NIH HHS/United States
- R21 HL085215-01/HL/NHLBI NIH HHS/United States
- R01 HL096652/HL/NHLBI NIH HHS/United States
- R01 HL70250/HL/NHLBI NIH HHS/United States
- R01-HL089598/HL/NHLBI NIH HHS/United States
- R01 HL091947/HL/NHLBI NIH HHS/United States
- R01 HL070250/HL/NHLBI NIH HHS/United States
- R01 HL089598/HL/NHLBI NIH HHS/United States
- T32 HL007706/HL/NHLBI NIH HHS/United States
- R01 HL117641/HL/NHLBI NIH HHS/United States
- T32-HL007706/HL/NHLBI NIH HHS/United States
- R01 HL079031/HL/NHLBI NIH HHS/United States
- R21 HL085215/HL/NHLBI NIH HHS/United States
- R01 HL62494/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

