Charge density-dependent modifications of hydration shell waters by Hofmeister ions
- PMID: 19603752
- PMCID: PMC2745343
- DOI: 10.1021/ja902240j
Charge density-dependent modifications of hydration shell waters by Hofmeister ions
Abstract
Gadolinium (Gd(3+)) vibronic sideband luminescence spectroscopy (GVSBLS) is used to probe, as a function of added Hofmeister series salts, changes in the OH stretching frequency derived from first-shell waters of aqueous Gd(3+) and of Gd(3+) coordinated to three different types of molecules: (i) a chelate (EDTA), (ii) structured peptides (mSE3/SE2) of the lanthanide-binding tags (LBTs) family with a single high-affinity binding site, and (iii) a calcium-binding protein (calmodulin) with four binding sites. The vibronic sideband (VSB) corresponding to the OH stretching mode of waters coordinated to Gd(3+), whose frequency is inversely correlated with the strength of the hydrogen bonding to neighboring waters, exhibits an increase in frequency when Gd(3+) becomes coordinated to either EDTA, calmodulin, or mSE3 peptide. In all of these cases, the addition of cation chloride or acetate salts to the solution increases the frequency of the vibronic band originating from the OH stretching mode of the coordinated waters in a cation- and concentration-dependent fashion. The cation dependence of the frequency increase scales with charge density of the cations, giving rise to an ordering consistent with the Hofmeister ordering. On the other hand, water Raman spectroscopy shows no significant change upon addition of these salts. Additionally, it is shown that the cation effect is modulated by the specific anion used. The results indicate a mechanism of action for Hofmeister series ions in which hydrogen bonding among hydration shell waters is modulated by several factors. High charge density cations sequester waters in a configuration that precludes strong hydrogen bonding to neighboring waters. Under such conditions, anion effects emerge as anions compete for hydrogen-bonding sites with the remaining free waters on the surface of the hydration shell. The magnitude of the anion effect is both cation and Gd(3+)-binding site specific.
Figures


 ) and NaCl (
) and NaCl ( ); and ii) Raman from a solution of 0.5 M Gd3+ in aqueous solution with: MgCl2 (
); and ii) Raman from a solution of 0.5 M Gd3+ in aqueous solution with: MgCl2 ( ) and NaCl (
) and NaCl ( ).
).
 ) and presence of 4.0 M MgCl2 (
) and presence of 4.0 M MgCl2 ( ). Inset: normalized –OH stretching mode VSB
). Inset: normalized –OH stretching mode VSB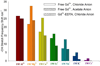
 0.5 M GdCl3 with chloride salts;
0.5 M GdCl3 with chloride salts;  0.5 M GdCl3 with acetate salts and
0.5 M GdCl3 with acetate salts and  80mM EDTA-Gd3+ with chloride salts.
80mM EDTA-Gd3+ with chloride salts.
 0.5 M Gd3+ ;
0.5 M Gd3+ ;  100 mM EDTA with 80 mM Gd3+ in 10 mM HEPES buffer at pH 7.0;
100 mM EDTA with 80 mM Gd3+ in 10 mM HEPES buffer at pH 7.0;  1 mM Calmodulin and 1 mM Gd3+ in 10 mM HEPES buffer at pH 7.0 and
1 mM Calmodulin and 1 mM Gd3+ in 10 mM HEPES buffer at pH 7.0 and  1 mM mSE3 and 1 mM Gd3+ in 10 mM HEPES buffer at pH 7.0.
1 mM mSE3 and 1 mM Gd3+ in 10 mM HEPES buffer at pH 7.0.  Bar graph of –OH stretching mode vibration frequency derived from GVSBLS of hydrated GdCl3 powder heated for 5 hours. b). Bar graph of –OH stretching mode vibration frequency derived from GVSBLS as a function of added NaCl for the following samples:
Bar graph of –OH stretching mode vibration frequency derived from GVSBLS of hydrated GdCl3 powder heated for 5 hours. b). Bar graph of –OH stretching mode vibration frequency derived from GVSBLS as a function of added NaCl for the following samples:  0.5 M Gd3+;
0.5 M Gd3+;  100 mM EDTA with 80 mM Gd3+ in 10 mM HEPES buffer at pH 7.0;
100 mM EDTA with 80 mM Gd3+ in 10 mM HEPES buffer at pH 7.0;  1 mM Calmodulin and 1 mM Gd3+ in 10 mM HEPES buffer at pH 7.0 and
1 mM Calmodulin and 1 mM Gd3+ in 10 mM HEPES buffer at pH 7.0 and  1 mM mSE3 and 1 mM Gd3+ in 10 mM HEPES buffer at pH 7.0 with varied concentration of NaCl.
1 mM mSE3 and 1 mM Gd3+ in 10 mM HEPES buffer at pH 7.0 with varied concentration of NaCl.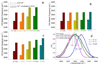
 absence of salts and presence of
absence of salts and presence of  Saturated MgF2,
Saturated MgF2,  1 M MgCl2 and
1 M MgCl2 and  1 M MgBr2, b)
1 M MgBr2, b)  absence of salts and presence of
absence of salts and presence of  1 M KF,
1 M KF,  1 M KCl,
1 M KCl,  1 M KBr and
1 M KBr and  1 M KI amd c)
1 M KI amd c)  absence of salts and presence of
absence of salts and presence of  1 M NaF,
1 M NaF,  1 M NaCl,
1 M NaCl,  1 M NaBr and
1 M NaBr and  1 M NaI. d) Plot of the frequency shift of –OH stretching mode vibration derived from GVSBLS of 0.25 M GdCl3 in trehalose glassy matrices with
1 M NaI. d) Plot of the frequency shift of –OH stretching mode vibration derived from GVSBLS of 0.25 M GdCl3 in trehalose glassy matrices with  absence and presence of
absence and presence of  MgCl2,
MgCl2,  NaCl,
NaCl,  NaF. (The original concentration of salts is 0.5 M.)
NaF. (The original concentration of salts is 0.5 M.)
References
-
- Hofmeister F. Arch. exp. Pathol. Pharmakol. 1888;24:247–260.
-
- Collins KD, Washabaugh MW. Q Rev Biophys. 1985;18:323–422. - PubMed
-
- Zhang Y, Cremer PS. Curr Opin Chem Biol. 2006;10:658–663. - PubMed
-
- Wilson EK. Chemical & Engineering News. 2007;85:47–49.
-
- Cacace MG, Landau EM, Ramsden JJ. Q Rev Biophys. 1997;30:241–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

