Up-regulation of integrin beta3 in radioresistant pancreatic cancer impairs adenovirus-mediated gene therapy
- PMID: 19604247
- PMCID: PMC11158494
- DOI: 10.1111/j.1349-7006.2009.01245.x
Up-regulation of integrin beta3 in radioresistant pancreatic cancer impairs adenovirus-mediated gene therapy
Abstract
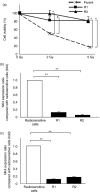
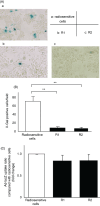
Adenovirus-mediated gene therapy is a promising approach for the treatment of pancreatic cancer. We previously reported that radiation enhanced adenovirus-mediated gene expression in pancreatic cancer, suggesting that adenoviral gene therapy might be more effective in radioresistant pancreatic cancer cells. In the present study, we compared the transduction efficiency of adenovirus-delivered genes in radiosensitive and radioresistant cells, and investigated the underlying mechanisms. We used an adenovirus expressing the hepatocyte growth factor antagonist, NK4 (Ad-NK4), as a representative gene therapy. We established two radioresistant human pancreatic cancer cell lines using fractionated irradiation. Radiosensitive and radioresistant pancreatic cancer cells were infected with Ad-NK4, and NK4 levels in the cells were measured. In order to investigate the mechanisms responsible for the differences in the transduction efficiency between these cells, we measured expression of the genes mediating adenovirus infection and endocytosis. The results revealed that NK4 levels in radioresistant cells were significantly lower (P < 0.01) than those in radiosensitive cells, although there were no significant differences in adenovirus uptake between radiosensitive cells and radioresistant cells. Integrin beta3 was up-regulated and the Coxsackie virus and adenovirus receptor was down-regulated in radioresistant cells, and inhibition of integrin beta3 promoted adenovirus gene transfer. These results suggest that inhibition of integrin beta3 in radioresistant pancreatic cancer cells could enhance adenovirus-mediated gene therapy.
Figures

Similar articles
-
Radiation enhances adenoviral gene therapy in pancreatic cancer via activation of cytomegalovirus promoter and increased adenovirus uptake.Clin Cancer Res. 2008 Mar 15;14(6):1859-67. doi: 10.1158/1078-0432.CCR-07-0933. Clin Cancer Res. 2008. PMID: 18347189
-
Chemotherapeutic agents potentiate adenoviral gene therapy for pancreatic cancer.Cancer Sci. 2009 Apr;100(4):722-9. doi: 10.1111/j.1349-7006.2009.01101.x. Epub 2009 Mar 11. Cancer Sci. 2009. PMID: 19302285 Free PMC article.
-
Gemcitabine synergistically enhances the effect of adenovirus gene therapy through activation of the CMV promoter in pancreatic cancer cells.Cancer Gene Ther. 2010 Aug;17(8):541-9. doi: 10.1038/cgt.2010.9. Epub 2010 Apr 16. Cancer Gene Ther. 2010. PMID: 20395979
-
Gene therapy for pancreatic cancer targeting the genomic alterations of tumor suppressor genes using replication-selective oncolytic adenovirus.Hum Cell. 2002 Sep;15(3):138-50. doi: 10.1111/j.1749-0774.2002.tb00108.x. Hum Cell. 2002. PMID: 12703544 Review.
-
Development of gene therapy to target pancreatic cancer.Cancer Sci. 2004 Apr;95(4):283-9. doi: 10.1111/j.1349-7006.2004.tb03204.x. Cancer Sci. 2004. PMID: 15072584 Free PMC article. Review.
Cited by
-
Tumor radiosensitization by monomethyl auristatin E: mechanism of action and targeted delivery.Cancer Res. 2015 Apr 1;75(7):1376-1387. doi: 10.1158/0008-5472.CAN-14-1931. Epub 2015 Feb 13. Cancer Res. 2015. PMID: 25681274 Free PMC article.
References
-
- Gunzburg WH, Salmons B. Novel clinical strategies for the treatment of pancreatic carcinoma. Trends Mol Med 2001; 7: 30–7. - PubMed
-
- Warshaw AL, Fernandez‐del Castillo C. Pancreatic carcinoma. N Eng J Med 1992; 326: 455–65. - PubMed
-
- Bramhall SR, Allum WH, Jones AG, Allwood A, Cummins C, Neoptolemos JP. Treatment and survival in 13 560 patients with pancreatic cancer, and incidence of the disease, in the West Midlands: an epidemiological study. Br J Surg 1995; 82: 111–5. - PubMed
-
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nature Rev 2002; 2: 897–909. - PubMed
-
- Jaffee EM, Hruban RH, Canto M, Kern SE. Focus on pancreas cancer. Cancer Cell 2002; 2: 25–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

