Optimally timing primaquine treatment to reduce Plasmodium falciparum transmission in low endemicity Thai-Myanmar border populations
- PMID: 19604346
- PMCID: PMC2718908
- DOI: 10.1186/1475-2875-8-159
Optimally timing primaquine treatment to reduce Plasmodium falciparum transmission in low endemicity Thai-Myanmar border populations
Abstract
Background: Effective malaria control has successfully reduced the malaria burden in many countries, but to eliminate malaria, these countries will need to further improve their control efforts. Here, a malaria control programme was critically evaluated in a very low-endemicity Thai-Myanmar border population, where early detection and prompt treatment have substantially reduced, though not ended, Plasmodium falciparum transmission, in part due to carriage of late-maturing gametocytes that remain post-treatment. To counter this effect, the WHO recommends the use of a single oral dose of primaquine along with an effective blood schizonticide. However, while the effectiveness of primaquine as a gametocidal agent is widely documented, the mismatch between primaquine's short half-life, the long-delay for gametocyte maturation and the proper timing of primaquine administration have not been studied.
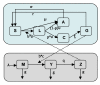
Methods: Mathematical models were constructed to simulate 8-year surveillance data, between 1999 and 2006, of seven villages along the Thai-Myanmar border. A simple model was developed to consider primaquine pharmacokinetics and pharmacodynamics, gametocyte carriage, and infectivity.
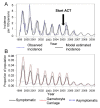
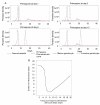
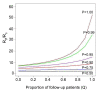
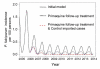
Results: In these populations, transmission intensity is very low, so the P. falciparum parasite rate is strongly linked to imported malaria and to the fraction of cases not treated. Given a 3.6-day half-life of gametocyte, the estimated duration of infectiousness would be reduced by 10 days for every 10-fold reduction in initial gametocyte densities. Infectiousness from mature gametocytes would last two to four weeks and sustain some transmission, depending on the initial parasite densities, but the residual mature gametocytes could be eliminated by primaquine. Because of the short half-life of primaquine (approximately eight hours), it was immediately obvious that with early administration (within three days after an acute attack), primaquine would not be present when mature gametocytes emerged eight days after the appearance of asexual blood-stage parasites. A model of optimal timing suggests that primaquine follow-up approximately eight days after a clinical episode could further reduce the duration of infectiousness from two to four weeks down to a few days. The prospects of malaria elimination would be substantially improved by changing the timing of primaquine administration and combining this with effective detection and management of imported malaria cases. The value of using primaquine to reduce residual gametocyte densities and to reduce malaria transmission was considered in the context of a malaria transmission model; the added benefit of the primaquine follow-up treatment would be relatively large only if a high fraction of patients (>95%) are initially treated with schizonticidal agents.
Conclusion: Mathematical models have previously identified the long duration of P. falciparum asexual blood-stage infections as a critical point in maintaining malaria transmission, but infectiousness can persist for two to four weeks because of residual populations of mature gametocytes. Simulations from new models suggest that, in areas where a large fraction of malaria cases are treated, curing the asexual parasitaemia in a primary infection, and curing mature gametocyte infections with an eight-day follow-up treatment with primaquine have approximately the same proportional effects on reducing the infectious period. Changing the timing of primaquine administration would, in all likelihood, interrupt transmission in this area with very good health systems and with very low endemicity.
Figures


Similar articles
-
Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs.Malar J. 2010 May 24;9:136. doi: 10.1186/1475-2875-9-136. Malar J. 2010. PMID: 20497536 Free PMC article.
-
A randomized feasibility trial comparing four antimalarial drug regimens to induce Plasmodium falciparum gametocytemia in the controlled human malaria infection model.Elife. 2018 Feb 27;7:e31549. doi: 10.7554/eLife.31549. Elife. 2018. PMID: 29482720 Free PMC article. Clinical Trial.
-
Nonrandomized controlled trial of artesunate plus sulfadoxine-pyrimethamine with or without primaquine for preventing posttreatment circulation of Plasmodium falciparum gametocytes.Antimicrob Agents Chemother. 2013 Jul;57(7):2948-54. doi: 10.1128/AAC.00139-13. Epub 2013 Apr 15. Antimicrob Agents Chemother. 2013. PMID: 23587943 Free PMC article. Clinical Trial.
-
Long term primaquine administration to reduce Plasmodium falciparum gametocyte transmission in hypoendemic areas.Southeast Asian J Trop Med Public Health. 2010 Nov;41(6):1306-11. Southeast Asian J Trop Med Public Health. 2010. PMID: 21329302 Review.
-
Population biology and antimalarial resistance: The transmission of antimalarial drug resistance in Plasmodium falciparum.Acta Trop. 2005 Jun;94(3):230-40. doi: 10.1016/j.actatropica.2005.04.014. Acta Trop. 2005. PMID: 15878154 Review.
Cited by
-
Electrocardiographic safety evaluation of extended artemether-lumefantrine treatment in patients with uncomplicated Plasmodium falciparum malaria in Bagamoyo District, Tanzania.Malar J. 2020 Jul 14;19(1):250. doi: 10.1186/s12936-020-03309-2. Malar J. 2020. PMID: 32664948 Free PMC article. Clinical Trial.
-
Chemoprophylaxis of Tropical Infectious Diseases.Pharmaceuticals (Basel). 2010 May 18;3(5):1561-1575. doi: 10.3390/ph3051561. Pharmaceuticals (Basel). 2010. PMID: 27713318 Free PMC article. Review.
-
A sticky situation: the unexpected stability of malaria elimination.Philos Trans R Soc Lond B Biol Sci. 2013 Jun 24;368(1623):20120145. doi: 10.1098/rstb.2012.0145. Print 2013 Aug 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23798693 Free PMC article.
-
Parasite clearance, cure rate, post-treatment prophylaxis and safety of standard 3-day versus an extended 6-day treatment of artemether-lumefantrine and a single low-dose primaquine for uncomplicated Plasmodium falciparum malaria in Bagamoyo district, Tanzania: a randomized controlled trial.Malar J. 2020 Jun 23;19(1):216. doi: 10.1186/s12936-020-03287-5. Malar J. 2020. PMID: 32576258 Free PMC article. Clinical Trial.
-
Transmission-blocking strategies: the roadmap from laboratory bench to the community.Malar J. 2016 Feb 18;15:95. doi: 10.1186/s12936-016-1163-3. Malar J. 2016. PMID: 26888537 Free PMC article. Review.
References
-
- World Health Organization and UNICEF World malaria report 2005. Geneva. 2005.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

