Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo
- PMID: 19604493
- PMCID: PMC3039716
- DOI: 10.1016/j.immuni.2009.06.015
Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo
Abstract
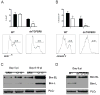
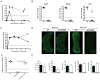
Although deficient CD8(+) T cell responses have long been associated with chronic viral infections, the underlying mechanisms are still unclear. Here we report that sustained transforming growth factor-beta (TGF-beta) expression and phosphorylation of its signaling mediator, Smad-2, were distinctive features of virus-specific CD8(+) T cells during chronic versus acute viral infections in vivo. The result was TGF-beta-dependent apoptosis of virus-specific CD8(+) T cells that related to upregulation of the proapoptotic protein Bim during chronic infection. Moreover, selective attenuation of TGF-beta signaling in T cells increased the numbers and multiple functions of antiviral CD8(+) T cells and enabled rapid eradication of the persistence-prone virus and memory generation. Finally, we found that cell-intrinsic TGF-beta signaling was responsible for virus-specific-CD8(+) T cell apoptosis and decreased numbers but was not necessary for their functional exhaustion. Our findings reveal persisting TGF-beta-Smad signaling as a hallmark and key regulator of CD8(+) T cell responses during chronic viral infections in vivo.
Figures




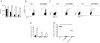
Similar articles
-
Id3 Controls Cell Death of 2B4+ Virus-Specific CD8+ T Cells in Chronic Viral Infection.J Immunol. 2015 Sep 1;195(5):2103-14. doi: 10.4049/jimmunol.1402607. Epub 2015 Jul 31. J Immunol. 2015. PMID: 26232435
-
Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction.Immunity. 2008 Feb;28(2):218-30. doi: 10.1016/j.immuni.2007.12.014. Immunity. 2008. PMID: 18275832
-
Role of Bim in regulating CD8+ T-cell responses during chronic viral infection.J Virol. 2006 Sep;80(17):8627-38. doi: 10.1128/JVI.00855-06. J Virol. 2006. PMID: 16912311 Free PMC article.
-
TGF-β blockade does not improve control of an established persistent viral infection.Viral Immunol. 2012 Jun;25(3):232-8. doi: 10.1089/vim.2011.0079. Epub 2012 May 23. Viral Immunol. 2012. PMID: 22620718 Free PMC article.
-
CD70-driven costimulation induces survival or Fas-mediated apoptosis of T cells depending on antigenic load.J Immunol. 2012 May 1;188(9):4256-67. doi: 10.4049/jimmunol.1102889. Epub 2012 Mar 26. J Immunol. 2012. PMID: 22450812
Cited by
-
T-cell exhaustion: understanding the interface of chronic viral and autoinflammatory diseases.Immunol Cell Biol. 2016 Nov;94(10):935-942. doi: 10.1038/icb.2016.81. Immunol Cell Biol. 2016. PMID: 27577866 Review.
-
The Effect of Unconventional Cytokine Combinations on NK-Cell Responses to Viral Infection.Front Immunol. 2021 Mar 19;12:645850. doi: 10.3389/fimmu.2021.645850. eCollection 2021. Front Immunol. 2021. PMID: 33815404 Free PMC article. Review.
-
The Role of ZEB2 in Human CD8 T Lymphocytes: Clinical and Cellular Immune Profiling in Mowat-Wilson Syndrome.Int J Mol Sci. 2021 May 18;22(10):5324. doi: 10.3390/ijms22105324. Int J Mol Sci. 2021. PMID: 34070208 Free PMC article.
-
TGF-β receptor maintains CD4 T helper cell identity during chronic viral infections.J Clin Invest. 2016 Oct 3;126(10):3799-3813. doi: 10.1172/JCI87041. Epub 2016 Sep 6. J Clin Invest. 2016. PMID: 27599295 Free PMC article.
-
Immunology and the elusive AIDS vaccine.Nature. 2010 Mar 11;464(7286):224-31. doi: 10.1038/nature08898. Nature. 2010. PMID: 20220841
References
-
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. - PubMed
-
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

