Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors
- PMID: 19605353
- PMCID: PMC2781998
- DOI: 10.1074/jbc.M109.014266
Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors
Abstract
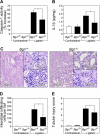
The role of endogenous inducers of inflammation is poorly understood. To produce the proinflammatory master cytokine interleukin (IL)-1beta, macrophages need double stimulation with ligands to both Toll-like receptors (TLRs) for IL-1beta gene transcription and nucleotide-binding oligomerization domain-like receptors for activation of the inflammasome. It is particularly intriguing to define how this complex regulation is mediated in the absence of an infectious trigger. Biglycan, a ubiquitous leucine-rich repeat proteoglycan of the extracellular matrix, interacts with TLR2/4 on macrophages. The objective of this study was to define the role of biglycan in the synthesis and activation of IL-1beta. Here we show that in macrophages, soluble biglycan induces the NLRP3/ASC inflammasome, activating caspase-1 and releasing mature IL-1beta without the need for additional costimulatory factors. This is brought about by the interaction of biglycan with TLR2/4 and purinergic P2X(4)/P2X(7) receptors, which induces receptor cooperativity. Furthermore, reactive oxygen species formation is involved in biglycan-mediated activation of the inflammasome. By signaling through TLR2/4, biglycan stimulates the expression of NLRP3 and pro-IL-1beta mRNA. Both in a model of non-infectious inflammatory renal injury (unilateral ureteral obstruction) and in lipopolysaccharide-induced sepsis, biglycan-deficient mice displayed lower levels of active caspase-1 and mature IL-1beta in the kidney, lung, and circulation. Our results provide evidence for direct activation of the NLRP3 inflammasome by biglycan and describe a fundamental paradigm of how tissue stress or injury is monitored by innate immune receptors detecting the release of the extracellular matrix components and turning such a signal into a robust inflammatory response.
Figures

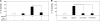
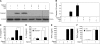

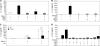



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

