Characterization of hepatitis C virus core protein multimerization and membrane envelopment: revelation of a cascade of core-membrane interactions
- PMID: 19605478
- PMCID: PMC2748039
- DOI: 10.1128/JVI.00066-09
Characterization of hepatitis C virus core protein multimerization and membrane envelopment: revelation of a cascade of core-membrane interactions
Abstract
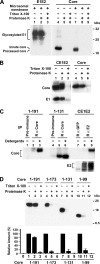
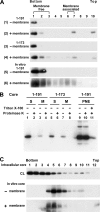
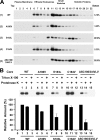
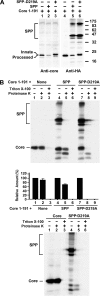
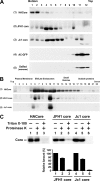
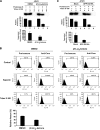
The molecular basis underlying hepatitis C virus (HCV) core protein maturation and morphogenesis remains elusive. We characterized the concerted events associated with core protein multimerization and interaction with membranes. Analyses of core proteins expressed from a subgenomic system showed that the signal sequence located between the core and envelope glycoprotein E1 is critical for core association with endoplasmic reticula (ER)/late endosomes and the core's envelopment by membranes, which was judged by the core's acquisition of resistance to proteinase K digestion. Despite exerting an inhibitory effect on the core's association with membranes, (Z-LL)(2)-ketone, a specific inhibitor of signal peptide peptidase (SPP), did not affect core multimeric complex formation, suggesting that oligomeric core complex formation proceeds prior to or upon core attachment to membranes. Protease-resistant core complexes that contained both innate and processed proteins were detected in the presence of (Z-LL)(2)-ketone, implying that core envelopment occurs after intramembrane cleavage. Mutations of the core that prevent signal peptide cleavage or coexpression with an SPP loss-of-function D219A mutant decreased the core's envelopment, demonstrating that SPP-mediated cleavage is required for core envelopment. Analyses of core mutants with a deletion in domain I revealed that this domain contains sequences crucial for core envelopment. The core proteins expressed by infectious JFH1 and Jc1 RNAs in Huh7 cells also assembled into a multimeric complex, associated with ER/late-endosomal membranes, and were enveloped by membranes. Treatment with (Z-LL)(2)-ketone or coexpression with D219A mutant SPP interfered with both core envelopment and infectious HCV production, indicating a critical role of core envelopment in HCV morphogenesis. The results provide mechanistic insights into the sequential and coordinated processes during the association of the HCV core protein with membranes in the early phase of virus maturation and morphogenesis.
Figures




Similar articles
-
Maturation of hepatitis C virus core protein by signal peptide peptidase is required for virus production.J Biol Chem. 2008 Jun 13;283(24):16850-9. doi: 10.1074/jbc.M802273200. Epub 2008 Apr 18. J Biol Chem. 2008. PMID: 18424431
-
Structural analysis of hepatitis C virus core-E1 signal peptide and requirements for cleavage of the genotype 3a signal sequence by signal peptide peptidase.J Virol. 2012 Aug;86(15):7818-28. doi: 10.1128/JVI.00457-12. Epub 2012 May 16. J Virol. 2012. PMID: 22593157 Free PMC article.
-
Intramembrane proteolysis and endoplasmic reticulum retention of hepatitis C virus core protein.J Virol. 2004 Jun;78(12):6370-80. doi: 10.1128/JVI.78.12.6370-6380.2004. J Virol. 2004. PMID: 15163730 Free PMC article.
-
Role of cleavage at the core-E1 junction of hepatitis C virus polyprotein in viral morphogenesis.PLoS One. 2017 Apr 24;12(4):e0175810. doi: 10.1371/journal.pone.0175810. eCollection 2017. PLoS One. 2017. PMID: 28437468 Free PMC article.
-
The potential of signal peptide peptidase as a therapeutic target for hepatitis C.Expert Opin Ther Targets. 2017 Sep;21(9):827-836. doi: 10.1080/14728222.2017.1369959. Epub 2017 Aug 22. Expert Opin Ther Targets. 2017. PMID: 28820612 Review.
Cited by
-
Human Choline Kinase-α Promotes Hepatitis C Virus RNA Replication through Modulation of Membranous Viral Replication Complex Formation.J Virol. 2016 Sep 29;90(20):9075-95. doi: 10.1128/JVI.00960-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27489281 Free PMC article.
-
Core as a novel viral target for hepatitis C drugs.Viruses. 2010 Aug;2(8):1734-1751. doi: 10.3390/v2081734. Epub 2010 Aug 20. Viruses. 2010. PMID: 21994704 Free PMC article.
-
Identification of Novel Functions for Hepatitis C Virus Envelope Glycoprotein E1 in Virus Entry and Assembly.J Virol. 2017 Mar 29;91(8):e00048-17. doi: 10.1128/JVI.00048-17. Print 2017 Apr 15. J Virol. 2017. PMID: 28179528 Free PMC article.
-
Genetic analysis of the carboxy-terminal region of the hepatitis C virus core protein.J Virol. 2010 Feb;84(4):1666-73. doi: 10.1128/JVI.02043-09. Epub 2009 Dec 9. J Virol. 2010. PMID: 20007277 Free PMC article.
-
HCV core residues critical for infectivity are also involved in core-NS5A complex formation.PLoS One. 2014 Feb 12;9(2):e88866. doi: 10.1371/journal.pone.0088866. eCollection 2014. PLoS One. 2014. PMID: 24533158 Free PMC article.
References
-
- Acosta-Rivero, N., A. Musacchio, L. Lorenzo, C. Alvarez, and J. Morales. 2002. Processing of the hepatitis C virus precursor protein expressed in the methylotrophic yeast Pichia pastoris. Biochem. Biophys. Res. Commun. 295:81-84. - PubMed
-
- Acosta-Rivero, N., A. Rodriguez, A. Musacchio, V. Falcon, V. M. Suarez, G. Martinez, I. Guerra, D. Paz-Lago, Y. Morera, M. C. de la Rosa, J. Morales-Grillo, and S. Duenas-Carrera. 2004. In vitro assembly into virus-like particles is an intrinsic quality of Pichia pastoris derived HCV core protein. Biochem. Biophys. Res. Commun. 325:68-74. - PubMed
-
- Barba, G., F. Harper, T. Harada, M. Kohara, S. Goulinet, Y. Matsuura, G. Eder, Z. Schaff, M. J. Chapman, T. Miyamura, and C. Brechot. 1997. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc. Natl. Acad. Sci. USA 94:1200-1205. - PMC - PubMed
-
- Bartenschlager, R., M. Frese, and T. Pietschmann. 2004. Novel insights into hepatitis C virus replication and persistence. Adv. Virus Res. 63:71-180. - PubMed
-
- Bartenschlager, R., and V. Lohmann. 2000. Replication of hepatitis C virus. J. Gen. Virol. 81:1631-1648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

