Artificial transmembrane oncoproteins smaller than the bovine papillomavirus E5 protein redefine sequence requirements for activation of the platelet-derived growth factor beta receptor
- PMID: 19605488
- PMCID: PMC2748040
- DOI: 10.1128/JVI.00946-09
Artificial transmembrane oncoproteins smaller than the bovine papillomavirus E5 protein redefine sequence requirements for activation of the platelet-derived growth factor beta receptor
Abstract
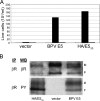
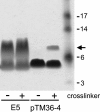
The bovine papillomavirus E5 protein (BPV E5) is a 44-amino-acid homodimeric transmembrane protein that binds directly to the transmembrane domain of the platelet-derived growth factor (PDGF) beta receptor and induces ligand-independent receptor activation. Three specific features of BPV E5 are considered important for its ability to activate the PDGF beta receptor and transform mouse fibroblasts: a pair of C-terminal cysteines, a transmembrane glutamine, and a juxtamembrane aspartic acid. By using a new genetic technique to screen libraries expressing artificial transmembrane proteins for activators of the PDGF beta receptor, we isolated much smaller proteins, from 32 to 36 residues, that lack all three of these features yet still dimerize noncovalently, specifically activate the PDGF beta receptor via its transmembrane domain, and transform cells efficiently. The primary amino acid sequence of BPV E5 is virtually unrecognizable in some of these proteins, which share as few as seven consecutive amino acids with the viral protein. Thus, small artificial proteins that bear little resemblance to a viral oncoprotein can nevertheless productively interact with the same cellular target. We speculate that similar cellular proteins may exist but have been overlooked due to their small size and hydrophobicity.
Figures






Similar articles
-
The bovine papillomavirus E5 protein and the PDGF beta receptor: it takes two to tango.Virology. 2009 Feb 20;384(2):345-51. doi: 10.1016/j.virol.2008.09.033. Epub 2008 Nov 6. Virology. 2009. PMID: 18990418 Free PMC article. Review.
-
Role of glutamine 17 of the bovine papillomavirus E5 protein in platelet-derived growth factor beta receptor activation and cell transformation.J Virol. 1998 Nov;72(11):8921-32. doi: 10.1128/JVI.72.11.8921-8932.1998. J Virol. 1998. PMID: 9765437 Free PMC article.
-
Molecular examination of the transmembrane requirements of the platelet-derived growth factor beta receptor for a productive interaction with the bovine papillomavirus E5 oncoprotein.J Biol Chem. 2002 Dec 6;277(49):47149-59. doi: 10.1074/jbc.M209582200. Epub 2002 Sep 25. J Biol Chem. 2002. PMID: 12351659
-
Specific locations of hydrophilic amino acids in constructed transmembrane ligands of the platelet-derived growth factor beta receptor.J Mol Biol. 2005 Jan 28;345(4):907-21. doi: 10.1016/j.jmb.2004.10.072. J Mol Biol. 2005. PMID: 15588835
-
The platelet-derived growth factor beta receptor as a target of the bovine papillomavirus E5 protein.Cytokine Growth Factor Rev. 2000 Dec;11(4):283-93. doi: 10.1016/s1359-6101(00)00012-5. Cytokine Growth Factor Rev. 2000. PMID: 10959076 Review.
Cited by
-
Compensatory mutants of the bovine papillomavirus E5 protein and the platelet-derived growth factor β receptor reveal a complex direct transmembrane interaction.J Virol. 2013 Oct;87(20):10936-45. doi: 10.1128/JVI.01475-13. Epub 2013 Aug 7. J Virol. 2013. PMID: 23926343 Free PMC article.
-
Biologically active LIL proteins built with minimal chemical diversity.Proc Natl Acad Sci U S A. 2015 Aug 25;112(34):E4717-25. doi: 10.1073/pnas.1514230112. Epub 2015 Aug 10. Proc Natl Acad Sci U S A. 2015. PMID: 26261320 Free PMC article.
-
Construction and maintenance of randomized retroviral expression libraries for transmembrane protein engineering.Protein Eng Des Sel. 2011 Mar;24(3):311-20. doi: 10.1093/protein/gzq112. Epub 2010 Dec 10. Protein Eng Des Sel. 2011. PMID: 21149273 Free PMC article.
-
Viral miniproteins.Annu Rev Microbiol. 2014;68:21-43. doi: 10.1146/annurev-micro-091313-103727. Epub 2014 Apr 10. Annu Rev Microbiol. 2014. PMID: 24742054 Free PMC article. Review.
-
The E5 proteins.Virology. 2013 Oct;445(1-2):99-114. doi: 10.1016/j.virol.2013.05.006. Epub 2013 May 31. Virology. 2013. PMID: 23731971 Free PMC article. Review.
References
-
- Adams, P. D., I. T. Arkin, D. M. Engelman, and A. T. Brunger. 1995. Computational searching and mutagenesis suggest a structure for the pentameric transmembrane domain of phospholamban. Nat. Struct. Biol. 2:154-162. - PubMed
-
- Adams, P. D., D. M. Engelman, and A. T. Brunger. 1996. Improved prediction of the structure of the dimeric transmembrane domain of glycophorin A obtained through global searching. Proteins 26:257-261. - PubMed
-
- Adduci, A. J., and R. Schlegel. 1999. The transmembrane domain of the E5 oncoprotein contains functionally discrete helical faces. J. Biol. Chem. 274:10249-10258. - PubMed
-
- Brunger, A. T., P. D. Adams, G. M. Clore, W. L. DeLano, P. Gros, R. W. Grosse-Kunstleve, J. S. Jiang, J. Kuszewski, M. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simonson, and G. L. Warren. 1998. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54:905-921. - PubMed
-
- Cohen, B. D., D. J. Goldstein, L. Rutledge, W. C. Vass, D. R. Lowy, R. Schlegel, and J. T. Schiller. 1993. Transformation-specific interaction of the bovine papillomavirus E5 oncoprotein with the platelet-derived growth factor receptor transmembrane domain and the epidermal growth factor receptor cytoplasmic domain. J. Virol. 67:5303-5311. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

