Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis
- PMID: 19605494
- PMCID: PMC2730408
- DOI: 10.1242/dev.038620
Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis
Abstract
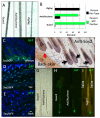
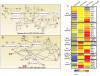
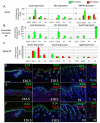
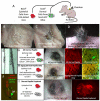
The dermal papilla comprises the specialised mesenchymal cells at the base of the hair follicle. Communication between dermal papilla cells and the overlying epithelium is essential for differentiation of the hair follicle lineages. We report that Sox2 is expressed in all dermal papillae at E16.5, but from E18.5 onwards expression is confined to a subset of dermal papillae. In postnatal skin, Sox2 is only expressed in the dermal papillae of guard/awl/auchene follicles, whereas CD133 is expressed both in guard/awl/auchene and in zigzag dermal papillae. Using transgenic mice that express GFP under the control of the Sox2 promoter, we isolated Sox2(+) (GFP(+)) CD133(+) cells and compared them with Sox2(-) (GFP(-)) CD133(+) dermal papilla cells. In addition to the 'core' dermal papilla gene signature, each subpopulation expressed distinct sets of genes. GFP(+) CD133(+) cells had upregulated Wnt, FGF and BMP pathways and expressed neural crest markers. In GFP(-) CD133(+) cells, the hedgehog, IGF, Notch and integrin pathways were prominent. In skin reconstitution assays, hair follicles failed to form when dermis was depleted of both GFP(+) CD133(+) and GFP(-) CD133(+) cells. In the absence of GFP(+) CD133(+) cells, awl/auchene hairs failed to form and only zigzag hairs were found. We have thus demonstrated a previously unrecognised heterogeneity in dermal papilla cells and shown that Sox2-positive cells specify particular hair follicle types.
Figures



References
-
- Botchkarev, V. A., Botchkareva, N. V., Nakamura, M., Huber, O., Funa, K., Lauster, R., Paus, R. and Gilchrest, B. A. (2001). Noggin is required for induction of the hair follicle growth phase in postnatal skin. FASEB J. 15, 2205-2214. - PubMed
-
- Botchkarev, V. A., Botchkareva, N. V., Sharov, A. A., Funa, K., Huber, O. and Gilchrest, B. A. (2002). Modulation of BMP signaling by noggin is required for induction of the secondary (nontylotrich) hair follicles. J. Invest. Dermatol. 118, 3-10. - PubMed
-
- Ehama, R., Ishimatsu-Tsuji, Y., Iriyama, S., Ideta, R., Soma, T., Yano, K., Kawasaki, C., Suzuki, S., Shirakata, Y., Hashimoto, K. et al. (2007). Hair follicle regeneration and human cells using grafted rodent and human cells. J. Invest. Dermatol. 127, 2106-2115. - PubMed
-
- Elliott, K., Stephenson, T. J. and Messenger, A. G. (1999). Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: implications for the control of hair follicle size and androgen responses. J. Invest. Dermatol. 113, 873-877. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

