Neutralization of vascular endothelial growth factor antiangiogenic isoforms is more effective than treatment with proangiogenic isoforms in stimulating vascular development and follicle progression in the perinatal rat ovary
- PMID: 19605786
- PMCID: PMC2770023
- DOI: 10.1095/biolreprod.109.078097
Neutralization of vascular endothelial growth factor antiangiogenic isoforms is more effective than treatment with proangiogenic isoforms in stimulating vascular development and follicle progression in the perinatal rat ovary
Abstract
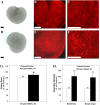
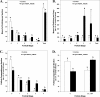
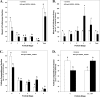
Inhibition of vascular endothelial growth factor A (VEGFA) signal transduction arrests vascular and follicle development. Because antiangiogenic VEGFA isoforms are proposed to block proangiogenic VEGFA isoforms from binding to their receptors, we hypothesized that proangiogenic isoforms promote and antiangiogenic isoforms inhibit these processes. The antiangiogenic isoforms Vegfa_165b and Vegfa_189b were amplified and sequenced from rat ovaries. The Vegfa_165b sequence was 90% homologous to human VEGFA_165B. Quantitative RT-PCR determined that Vegfa_165b mRNA was more abundant around Embryonic Day 18, but Vegfa_189b lacked a distinct pattern of abundance. Antiangiogenic VEGFA isoforms were localized to pregranulosa and granulosa cells of all follicle stages and to theca cells of advanced-stage follicles. To determine the effects of VEGFA isoforms in developing ovaries, Postnatal Day 3/4 rat ovaries were cultured with VEGFA_164 or an antibody to antiangiogenic isoforms (anti-VEGFAxxxB). Treatment with 50 ng/ml of VEGFA_164 resulted in a 93% increase in vascular density (P < 0.01), and treated ovaries were composed of fewer primordial follicles (stage 0) and more developing follicles (stages 1-4) than controls (P < 0.04). Ovaries treated with 5 ng/ml of VEGFAxxxB antibody had a 93% increase in vascular density (P < 0.02), with fewer primordial and early primary follicles (stage 1) and more primary, transitional, and secondary follicles (stages 2, 3, and 4, respectively) compared with controls (P < 0.005). We conclude that neutralization of antiangiogenic VEGFA isoforms may be a more effective mechanism of enhancing vascular and follicular development in perinatal rat ovaries than treatment with the proangiogenic isoform VEGFA_164.
Figures



References
-
- Hirshfield AN.Heterogeneity of cell populations that contribute to the formation of primordial follicles in rats. Biol Reprod 1992; 47: 466–472.. - PubMed
-
- Rajah R, Glaser EM, Hirshfield AN.The changing architecture of the neonatal rat ovary during histogenesis. Dev Dyn 1992; 194: 177–192.. - PubMed
-
- Pepling ME, Spradling AC.Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 2001; 234: 339–351.. - PubMed
-
- Nilsson E, Skinner MK.Cellular interactions that control primordial follicle development and folliculogenesis. J Soc Gynecol Investig 2001; 8: S17–S20.. - PubMed
-
- Parrott JA, Skinner MK.Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology 1999; 140: 4262–4271.. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

