Disclosure of APOE genotype for risk of Alzheimer's disease
- PMID: 19605829
- PMCID: PMC2778270
- DOI: 10.1056/NEJMoa0809578
Disclosure of APOE genotype for risk of Alzheimer's disease
Abstract
Background: The apolipoprotein E (APOE) genotype provides information on the risk of Alzheimer's disease, but the genotyping of patients and their family members has been discouraged. We examined the effect of genotype disclosure in a prospective, randomized, controlled trial.
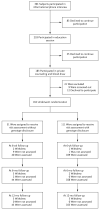
Methods: We randomly assigned 162 asymptomatic adults who had a parent with Alzheimer's disease to receive the results of their own APOE genotyping (disclosure group) or not to receive such results (nondisclosure group). We measured symptoms of anxiety, depression, and test-related distress 6 weeks, 6 months, and 1 year after disclosure or nondisclosure.
Results: There were no significant differences between the two groups in changes in time-averaged measures of anxiety (4.5 in the disclosure group and 4.4 in the nondisclosure group, P=0.84), depression (8.8 and 8.7, respectively; P=0.98), or test-related distress (6.9 and 7.5, respectively; P=0.61). Secondary comparisons between the nondisclosure group and a disclosure subgroup of subjects carrying the APOE epsilon4 allele (which is associated with increased risk) also revealed no significant differences. However, the epsilon4-negative subgroup had a significantly lower level of test-related distress than did the epsilon4-positive subgroup (P=0.01). Subjects with clinically meaningful changes in psychological outcomes were distributed evenly among the nondisclosure group and the epsilon4-positive and epsilon4-negative subgroups. Baseline scores for anxiety and depression were strongly associated with post-disclosure scores of these measures (P<0.001 for both comparisons).
Conclusions: The disclosure of APOE genotyping results to adult children of patients with Alzheimer's disease did not result in significant short-term psychological risks. Test-related distress was reduced among those who learned that they were APOE epsilon4-negative. Persons with high levels of emotional distress before undergoing genetic testing were more likely to have emotional difficulties after disclosure. (ClinicalTrials.gov number, NCT00571025.)
2009 Massachusetts Medical Society
Figures
Comment in
-
Effect of genetic testing for risk of Alzheimer's disease.N Engl J Med. 2009 Jul 16;361(3):298-9. doi: 10.1056/NEJMe0903449. N Engl J Med. 2009. PMID: 19605835 No abstract available.
-
ACP Journal Club: Disclosure of genetic risk for Alzheimer disease did not increase anxiety or depression in asymptomatic adults.Ann Intern Med. 2009 Nov 17;151(10):JC5-9. doi: 10.7326/0003-4819-151-10-200911170-02009. Ann Intern Med. 2009. PMID: 19920267 No abstract available.
-
Disclosure of the genetic risk of Alzheimer's disease.N Engl J Med. 2010 Jan 14;362(2):181-2; author reply 182. doi: 10.1056/NEJMc096300. N Engl J Med. 2010. PMID: 20071713 No abstract available.
References
-
- Wolfberg AJ. Genes on the Web — direct-to-consumer marketing of genetic testing. N Engl J Med. 2006;355:543–5. - PubMed
-
- Hunter DJ, Khoury MJ, Drazen JM. Letting the genome out of the bottle — will we get our wish? N Engl J Med. 2008;358:105–7. - PubMed
-
- Offit K. Genomic profiles for disease risk: predictive or premature? JAMA. 2008;299:1353–5. - PubMed
-
- Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349–56. - PubMed
-
- Farrer LA, Brin MF, Elsas L, et al. Statement on use of apolipoprotein E testing for Alzheimer disease. JAMA. 1995;274:1627–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous