Nanomechanical in situ monitoring of proteolysis of peptide by Cathepsin B
- PMID: 19606222
- PMCID: PMC2707113
- DOI: 10.1371/journal.pone.0006248
Nanomechanical in situ monitoring of proteolysis of peptide by Cathepsin B
Abstract
Characterization and control of proteolysis of peptides by specific cellular protease is a priori requisite for effective drug discovery. Here, we report the nanomechanical, in situ monitoring of proteolysis of peptide chain attributed to protease (Cathepsin B) by using a resonant nanomechanical microcantilever immersed in a liquid. Specifically, the detection is based on measurement of resonant frequency shift arising from proteolysis of peptides (leading to decrease of cantilever's overall mass, and consequently, increases in the resonance). It is shown that resonant microcantilever enables the quantification of proteolysis efficacy with respect to protease concentration. Remarkably, the nanomechanical, in situ monitoring of proteolysis allows us to gain insight into the kinetics of proteolysis of peptides, which is well depicted by Langmuir kinetic model. This implies that nanomechanical biosensor enables the characterization of specific cellular protease such as its kinetics.
Conflict of interest statement
Figures

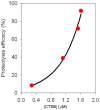
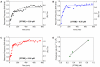
References
-
- Kodadek T. Biochemistry: Molecular cloaking devices. Nature. 2008;453:861–862. - PubMed
-
- Yang J, Lee C-H, Ko H-J, Suh J-S, Yoon H-G, et al. Multifunctional Magneto-Polymeric Nanohybrids for Targeted Detection and Synergistic Therapeutic Effects on Breast Cancer13. Angew Chem Int Ed. 2007;46:8836–8839. - PubMed
-
- Defoe-Jones D, Garsky VM, Wong BK, Feng D-M, Bolyar T, et al. A peptide-doxorubicin ‘prodrug’ activated prostate-specific antigen selectively kills prostate tumor cells positive for prostate-specific antigen in vivo. Nature Medicine. 2000;6:1248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

