Genome analysis and expression patterns of odorant-binding proteins from the Southern House mosquito Culex pipiens quinquefasciatus
- PMID: 19606229
- PMCID: PMC2707629
- DOI: 10.1371/journal.pone.0006237
Genome analysis and expression patterns of odorant-binding proteins from the Southern House mosquito Culex pipiens quinquefasciatus
Abstract
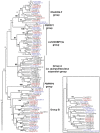
Olfactory-based behaviors in mosquitoes are mediated by odorant-binding proteins (OBPs). They form a multigenic family involved in the peripheral events in insect olfaction, specifically the transport of odorants to membrane-bound odorant receptors. OBPs contribute to the remarkable sensitivity of the insect's olfactory system and may be involved in the selective transport of odorants.We have employed a combination of bioinformatics and molecular approaches to identify and characterize members of the "classic" OBP family in the Southern House mosquito Culex pipiens quinquefasciatus ( = Cx. quinquefasciatus), a vector of pathogens causing several human diseases. By taking advantage of the recently released genome sequences, we have identified fifty-three putative Cx. quinquefasciatus OBP genes by Blast searches. As a first step towards their molecular characterization, expression patterns by RT-PCR revealed thirteen genes that were detected exclusively and abundantly in chemosensory tissues. No clear differences were observed in the transcripts levels of olfactory-specific OBPs between antennae of both sexes using semi-quantitative RT-PCR. Phylogenetic and comparative analysis revealed orthologous of Cx. quinquefasciatus OBPs in Anopheles gambiae and Aedes aegypti. The identification of fifty-three putative OBP genes in Cx. quinquefasciatus highlights the diversity of this family. Tissue-specificity study suggests the existence of different functional classes within the mosquito OBP family. Most genes were detected in chemosensory as well as non chemosensory tissues indicating that they might be encapsulins, but not necessarily olfactory proteins. On the other hand, thirteen "true" OBP genes were detected exclusively in olfactory tissues and might be involved specifically in the detection of "key" semiochemicals. Interestingly, in Cx. quinquefasciatus olfactory-specific OBPs belong exclusively to four distinct phylogenetic groups which are particularly well conserved among three mosquito species.
Conflict of interest statement
Figures



Similar articles
-
Characterization of olfactory genes in the antennae of the Southern house mosquito, Culex quinquefasciatus.J Insect Physiol. 2011 Jul;57(7):915-29. doi: 10.1016/j.jinsphys.2011.04.003. Epub 2011 Apr 9. J Insect Physiol. 2011. PMID: 21504749
-
Comparative genomics of odorant binding proteins in Anopheles gambiae, Aedes aegypti, and Culex quinquefasciatus.Genome Biol Evol. 2013;5(1):163-80. doi: 10.1093/gbe/evs131. Genome Biol Evol. 2013. PMID: 23292137 Free PMC article.
-
Screening of olfactory genes related to blood-feeding behaviors in Culex pipiens quinquefasciatus and Culex pipiens molestus by transcriptome analysis.PLoS Negl Trop Dis. 2022 Feb 7;16(2):e0010204. doi: 10.1371/journal.pntd.0010204. eCollection 2022 Feb. PLoS Negl Trop Dis. 2022. PMID: 35130307 Free PMC article.
-
Molecular and functional characterization of odorant-binding protein genes in an invasive vector mosquito, Aedes albopictus.PLoS One. 2013 Jul 23;8(7):e68836. doi: 10.1371/journal.pone.0068836. Print 2013. PLoS One. 2013. PMID: 23935894 Free PMC article.
-
Enantiomeric Discrimination in Insects: The Role of OBPs and ORs.Insects. 2022 Apr 8;13(4):368. doi: 10.3390/insects13040368. Insects. 2022. PMID: 35447810 Free PMC article. Review.
Cited by
-
Dengue virus infection of the Aedes aegypti salivary gland and chemosensory apparatus induces genes that modulate infection and blood-feeding behavior.PLoS Pathog. 2012;8(3):e1002631. doi: 10.1371/journal.ppat.1002631. Epub 2012 Mar 29. PLoS Pathog. 2012. PMID: 22479185 Free PMC article.
-
Fatty acid solubilizer from the oral disk of the blowfly.PLoS One. 2013;8(1):e51779. doi: 10.1371/journal.pone.0051779. Epub 2013 Jan 11. PLoS One. 2013. PMID: 23326317 Free PMC article.
-
Structure of an odorant-binding protein from the mosquito Aedes aegypti suggests a binding pocket covered by a pH-sensitive "Lid".PLoS One. 2009 Nov 26;4(11):e8006. doi: 10.1371/journal.pone.0008006. PLoS One. 2009. PMID: 19956631 Free PMC article.
-
Proteomic Analysis of Pig (Sus scrofa) Olfactory Soluble Proteome Reveals O-Linked-N-Acetylglucosaminylation of Secreted Odorant-Binding Proteins.Front Endocrinol (Lausanne). 2014 Dec 5;5:202. doi: 10.3389/fendo.2014.00202. eCollection 2014. Front Endocrinol (Lausanne). 2014. PMID: 25538681 Free PMC article.
-
Odorant Binding Proteins (OBPs) and Odorant Receptors (ORs) of Anopheles stephensi: Identification and comparative insights.PLoS One. 2022 Mar 22;17(3):e0265896. doi: 10.1371/journal.pone.0265896. eCollection 2022. PLoS One. 2022. PMID: 35316281 Free PMC article.
References
-
- Vogt RG, Riddiford LM. Pheromone binding and inactivation by moth antennae. Nature. 1981;293:161–163. - PubMed
-
- Leal WS. Pheromone reception. Top Curr Chem. 2005;240:1–36.
-
- Wojtasek H, Leal WS. Conformational change in the pheromone-binding protein from Bombyx mori induced by pH and by interaction with membranes. J Biol Chem. 1999;274:30950–30956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

