Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates
- PMID: 19607716
- PMCID: PMC2716374
- DOI: 10.1186/1471-2164-10-321
Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates
Abstract
Background: About 45% of the human genome is comprised of mobile transposable elements or "junk DNA". The exaptation or co-option of these elements to provide important cellular functions is hypothesized to have played a powerful force in evolution; however, proven examples are rare. An ancient primate-specific Alu short interspersed element (SINE) put the human CAMP gene under the regulation of the vitamin D pathway by providing a perfect vitamin D receptor binding element (VDRE) in its promoter. Subsequent studies demonstrated that the vitamin D-cathelicidin pathway may be a key component of a novel innate immune response of human to infection. The lack of evolutionary conservation in non-primate mammals suggested that this is a primate-specific adaptation. Evidence for evolutionary conservation of this regulation in additional primate lineages would provide strong evidence that the TLR2/1-vitamin D-cathelicidin pathway evolved as a biologically important immune response mechanism protecting human and non-human primates against infection.
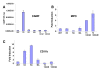
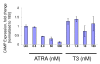
Results: PCR-based amplification of the Alu SINE from human and non-human primate genomic DNA and subsequent sequence analysis, revealed perfect structural conservation of the VDRE in all primates examined. Reporter gene studies and induction of the endogenous CAMP gene in Rhesus macaque peripheral blood mononuclear cells demonstrated that the VDREs were conserved functionally. In addition, New World monkeys (NWMs) have maintained additional, functional steroid-hormone receptor binding sites in the AluSx SINE that confer retinoic acid responsiveness and provide potential thyroid hormone receptor binding sites. These sites were less well-conserved during human, ape and Old World monkey (OWM) evolution and the human CAMP gene does not respond to either retinoic acid or thyroid hormone.
Conclusion: We demonstrated that the VDRE in the CAMP gene originated from the exaptation of an AluSx SINE in the lineage leading to humans, apes, OWMs and NWMs and remained under purifying selection for the last 55-60 million years. We present convincing evidence of an evolutionarily fixed, Alu-mediated divergence in steroid hormone nuclear receptor gene regulation between humans/primates and other mammals. Evolutionary selection to place the primate CAMP gene under regulation of the vitamin D pathway potentiates the innate immune response and may counter the anti-inflammatory properties of vitamin D.
Figures


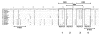
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

