Toxoplasma H2A variants reveal novel insights into nucleosome composition and functions for this histone family
- PMID: 19607843
- PMCID: PMC2734877
- DOI: 10.1016/j.jmb.2009.07.017
Toxoplasma H2A variants reveal novel insights into nucleosome composition and functions for this histone family
Abstract
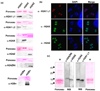
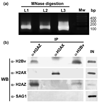
Toxoplasma gondii is an obligate intracellular parasite. Toxoplasmosis is incurable because of its ability to differentiate from the rapidly replicating tachyzoite stage into a latent cyst form (bradyzoite stage). Gene regulation pertinent to Toxoplasma differentiation involves histone modification, but very little is known about the histone proteins in this early branching eukaryote. Here, we report the characterization of three H2A histones, variants H2AX and H2AZ, and a canonical H2A1. H2AZ is the minor parasite H2A member. H2A1 and H2AX both have an SQ motif, but only H2AX has a complete SQ(E/D)varphi (where varphi denotes a hydrophobic residue) known to be phosphorylated in response to DNA damage. We show that a novel H2B variant interacts with H2AZ and H2A1 but not with H2AX. Chromatin immunoprecipitation (ChIP) revealed that H2AZ and H2Bv are enriched at active genes while H2AX is enriched at repressed genes as well as the silent TgIRE repeat element. During DNA damage, we detected an increase in H2AX phosphorylation as well as increases in h2a1 and h2ax transcription. We found that expression of h2ax, but not h2a1 or h2az, increases in bradyzoites generated in vitro. Similar analysis performed on mature bradyzoites generated in vivo, which are arrested in G0, showed that h2az and h2ax are expressed but h2a1 is not, consistent with the idea that h2a1 is the canonical histone orthologue in the parasite. The increase of H2AX, which localizes to silenced areas during bradyzoite differentiation, is consistent with the quiescent nature of this stage of the life cycle. Our results indicate that the early-branching eukaryotic parasite Toxoplasma contains nucleosomes of novel composition, which is likely to impact multiple facets of parasite biology, including the clinically important process of bradyzoite differentiation.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

