Gangliosides in cell recognition and membrane protein regulation
- PMID: 19608407
- PMCID: PMC2763983
- DOI: 10.1016/j.sbi.2009.06.001
Gangliosides in cell recognition and membrane protein regulation
Abstract
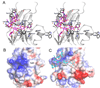
Gangliosides, sialic acid-bearing glycosphingolipids, are expressed on all vertebrate cells, and are the major glycans on nerve cells. They are anchored to the plasma membrane through their ceramide lipids with their varied glycans extending into the extracellular space. Through sugar-specific interactions with glycan-binding proteins on apposing cells, gangliosides function as receptors in cell-cell recognition, regulating natural killer cell cytotoxicity via Siglec-7, myelin-axon interactions via Siglec-4 (myelin-associated glycoprotein), and inflammation via E-selectin. Gangliosides also interact laterally in their own membranes, regulating the responsiveness of signaling proteins including the insulin, epidermal growth factor, and vascular endothelial growth factor receptors. In these ways, gangliosides act as regulatory elements in the immune system, in the nervous system, in metabolic regulation, and in cancer progression.
Figures

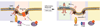

References
-
- Schnaar RL. Glycolipid-mediated cell-cell recognition in inflammation and nerve regeneration. Arch Biochem Biophys. 2004;426:163–172. - PubMed
-
- Sonnino S, Mauri L, Chigorno V, Prinetti A. Gangliosides as components of lipid membrane domains. Glycobiology. 2007;17:1R–13R. - PubMed
-
- Prinetti A, Loberto N, Chigorno V, Sonnino S. Glycosphingolipid behaviour in complex membranes. Biochim Biophys Acta. 2009;1788:184–193. - PubMed
-
- Imberty A, Varrot A. Microbial recognition of human cell surface glycoconjugates. Curr Opin Struct Biol. 2008;18:567–576. - PubMed
-
- Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

