Potential late-onset Alzheimer's disease-associated mutations in the ADAM10 gene attenuate {alpha}-secretase activity
- PMID: 19608551
- PMCID: PMC2748890
- DOI: 10.1093/hmg/ddp323
Potential late-onset Alzheimer's disease-associated mutations in the ADAM10 gene attenuate {alpha}-secretase activity
Abstract
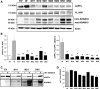
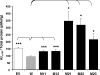
ADAM10, a member of a disintegrin and metalloprotease family, is an alpha-secretase capable of anti-amyloidogenic proteolysis of the amyloid precursor protein. Here, we present evidence for genetic association of ADAM10 with Alzheimer's disease (AD) as well as two rare potentially disease-associated non-synonymous mutations, Q170H and R181G, in the ADAM10 prodomain. These mutations were found in 11 of 16 affected individuals (average onset age 69.5 years) from seven late-onset AD families. Each mutation was also found in one unaffected subject implying incomplete penetrance. Functionally, both mutations significantly attenuated alpha-secretase activity of ADAM10 (>70% decrease), and elevated Abeta levels (1.5-3.5-fold) in cell-based studies. In summary, we provide the first evidence of ADAM10 as a candidate AD susceptibility gene, and report two potentially pathogenic mutations with incomplete penetrance for late-onset familial AD.
Figures


References
-
- Saitoh T., Sundsmo M., Roch J.M., Kimura N., Cole G., Schubert D., Oltersdorf T., Schenk D.B. Secreted form of amyloid beta protein precursor is involved in the growth regulation of fibroblasts. Cell. 1989;58:615–622. - PubMed
-
- Furukawa K., Sopher B.L., Rydel R.E., Begley J.G., Pham D.G., Martin G.M., Fox M., Mattson M.P. Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. J. Neurochem. 1996;67:1882–1896. - PubMed
-
- Mattson M.P., Guo Z.H., Geiger J.D. Secreted form of amyloid precursor protein enhances basal glucose and glutamate transport and protects against oxidative impairment of glucose and glutamate transport in synaptosomes by a cyclic GMP-mediated mechanism. J. Neurochem. 1999;73:532–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

