The integrin alpha9beta1 on hematopoietic stem and progenitor cells: involvement in cell adhesion, proliferation and differentiation
- PMID: 19608669
- PMCID: PMC2770959
- DOI: 10.3324/haematol.2009.006072
The integrin alpha9beta1 on hematopoietic stem and progenitor cells: involvement in cell adhesion, proliferation and differentiation
Abstract
Background: Hematopoietic stem and progenitor cells can interact with their microenvironment via integrins which are adhesion receptors consisting of alpha and beta subunits. Current knowledge suggests that the integrin subunits alpha4 and alpha6 expressed on hematopoietic stem and progenitor cells have distinct roles in retaining stem cells in the bone marrow. The aim of our study was to gain insight into the expression and functions of the integrin subunits alpha7-alpha11 within the endosteal stem cell niche.
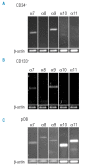
Design and methods: Human osteoblasts isolated from trabecular bone and hematopoietic stem and progenitor cells purified from umbilical cord blood or bone marrow aspirates were analyzed for the expression of integrin alpha7-alpha11 chains by reverse transcriptase polymerase chain reaction. The involvement of the integrin alpha9beta1 in hematopoietic stem and progenitor cell adhesion, proliferation and differentiation was analyzed in functional assays.
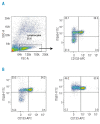
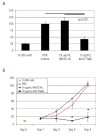
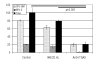
Results: Transcripts for all investigated integrin chains were found in primary osteoblasts. Highly purified hematopoietic stem and progenitor cells, however, expressed only transcripts encoding integrin subunits alpha7 and alpha9. Flow cytometric analysis verified extracellular expression of the integrin alpha9beta1 on hematopoietic stem and progenitor cells. Cell-cell adhesion assays with osteoblasts and dye-labeled CD34(+) hematopoietic stem and progenitor cells in the presence of function-blocking antibodies revealed a role of integrin alpha9 in hematopoietic stem and progenitor cell adhesion to osteoblasts. Furthermore, the addition of anti-integrin alpha9 antibodies significantly inhibited proliferation and in vitro differentiation of CD34(+) hematopoietic stem and progenitor cells.
Conclusions: The integrin alpha9beta1 has been identified as a new member of the integrin beta1-subfamily expressed on human hematopoietic stem and progenitor cells. The functional studies strongly suggest that integrin alpha9beta1 contributes to adhesion and differentiation of hematopoietic stem and progenitor cells in the endosteal stem cell niche.
Figures


Comment in
-
Parsing the niche code: the molecular mechanisms governing hematopoietic stem cell adhesion and differentiation.Haematologica. 2009 Nov;94(11):1477-81. doi: 10.3324/haematol.2009.013730. Haematologica. 2009. PMID: 19880773 Free PMC article. Review.
References
-
- Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. - PubMed
-
- Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. - PubMed
-
- Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–56. - PubMed
-
- Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

