Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway
- PMID: 19608737
- PMCID: PMC2757987
- DOI: 10.1074/jbc.M109.033472
Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway
Abstract
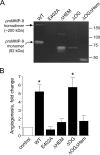
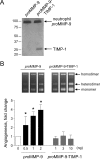
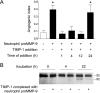
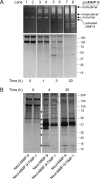
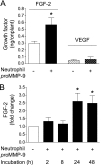
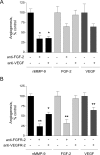
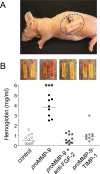
The structural and catalytic requirements for neutrophil MMP-9 proenzyme (proMMP-9) to induce angiogenesis were investigated using a quantitative angiogenesis model based on grafting of collagen onplants onto the chorioallantoic membrane of chick embryos. Both physiological activation of neutrophil proMMP-9 and proteolytic activity of the generated MMP-9 enzyme were critically dependent on the tissue inhibitor of metalloproteinase (TIMP)-free status of the zymogen. The presence of an intact active site and hemopexin domain were required for full angiogenesis-inducing activity of the MMP-9 enzyme. Timed additions of TIMP-1 to the onplants containing TIMP-free neutrophil proMMP-9 indicated that in vivo activation of the zymogen occurred during the first 24 h after grafting. Within the onplant tissue, MMP-9 activation was accompanied by proteolytic modifications of fibrillar collagen and an influx of host proteins, the rate of which depended on the TIMP-free status of the zymogen. By quantifying the levels of host angiogenic factors, we demonstrated that basic fibroblast growth factor (FGF-2) was a major cytokine becoming bioavailable in the onplant tissue undergoing a neutrophil proMMP-9-mediated angiogenic switch. Inhibition of angiogenesis with specific function-blocking antibodies further indicated an involvement of a FGF-2/FGFR-2 pathway in neutrophil proMMP-9-induced angiogenesis. The enhanced angiogenesis catalyzed by neutrophil MMP-9 appears to evoke also a localized, low threshold level vascular endothelial growth factor (VEGF)/VEGFR-2 pathway, likely functioning in the formation and/or stabilization of blood vessels. That neutrophil proMMP-9, unencumbered by TIMP-1, directly mediates FGF-2-dependent angiogenesis was also demonstrated in our quantitative mouse angiogenesis model employing subcutaneous collagen implants, thus implicating the novel TIMP-free MMP-9/FGF-2/FGFR-2 pathway in proMMP-9-induced angiogenesis in a mammalian setting.
Figures
References
-
- Vu T. H., Werb Z. (ed) (1998) in Matrix Metalloproteinases ( Parks W. C., Mecham R. P. eds) pp. 115–148, Academic Press, San Diego
-
- Opdenakker G., Van den Steen P. E., Dubois B., Nelissen I., Van Coillie E., Masure S., Proost P., Van Damme J. (2001) J. Leukocyte Biol. 69, 851–859 - PubMed
-
- Van den Steen P. E., Dubois B., Nelissen I., Rudd P. M., Dwek R. A., Opdenakker G. (2002) Crit. Rev. Biochem. Mol. Biol. 37, 375–536 - PubMed
-
- Parks W. C., Wilson C. L., López-Boado Y. S. (2004) Nat. Rev. Immunol. 4, 617–629 - PubMed
-
- Björklund M., Koivunen E. (2005) Biochim. Biophys. Acta 1755, 37–69 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

