Protein kinase A increases type-2 inositol 1,4,5-trisphosphate receptor activity by phosphorylation of serine 937
- PMID: 19608738
- PMCID: PMC2757215
- DOI: 10.1074/jbc.M109.010132
Protein kinase A increases type-2 inositol 1,4,5-trisphosphate receptor activity by phosphorylation of serine 937
Abstract
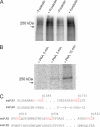
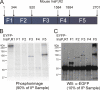
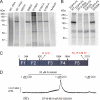
Protein kinase A (PKA) phosphorylation of inositol 1,4,5-trisphosphate receptors (InsP(3)Rs) represents a mechanism for shaping intracellular Ca(2+) signals following a concomitant elevation in cAMP. Activation of PKA results in enhanced Ca(2+) release in cells that express predominantly InsP(3)R2. PKA is known to phosphorylate InsP(3)R2, but the molecular determinants of this effect are not known. We have expressed mouse InsP(3)R2 in DT40-3KO cells that are devoid of endogenous InsP(3)R and examined the effects of PKA phosphorylation on this isoform in unambiguous isolation. Activation of PKA increased Ca(2+) signals and augmented the single channel open probability of InsP(3)R2. A PKA phosphorylation site unique to the InsP(3)R2 was identified at Ser(937). The enhancing effects of PKA activation on this isoform required the phosphorylation of Ser(937), since replacing this residue with alanine eliminated the positive effects of PKA activation. These results provide a mechanism responsible for the enhanced Ca(2+) signaling following PKA activation in cells that express predominantly InsP(3)R2.
Figures




References
-
- Berridge M. J. (1993) Nature 361, 315–325 - PubMed
-
- Bruce J. I., Straub S. V., Yule D. I. (2003) Cell Calcium 34, 431–444 - PubMed
-
- Natarajan M., Lin K. M., Hsueh R. C., Sternweis P. C., Ranganathan R. (2006) Nat. Cell Biol. 8, 571–580 - PubMed
-
- Bruce J. I., Shuttleworth T. J., Giovannucci D. R., Yule D. I. (2002) J. Biol. Chem. 277, 1340–1348 - PubMed
-
- Straub S. V., Wagner L. E., 2nd, Bruce J. I., Yule D. I. (2004) Biol. Res. 37, 593–602 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

