Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation
- PMID: 19608970
- PMCID: PMC2753598
- DOI: 10.1161/ATVBAHA.109.192658
Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation
Abstract
Objective: Obesity promotes macrophage infiltration into adipose tissue and is associated with increases in several cardiovascular diseases. Infusion of angiotensin II (AngII) to mice induces formation of abdominal aortic aneurysms (AAAs) with profound medial and adventitial macrophage infiltration. We sought to determine whether obesity promotes macrophage infiltration and proinflammatory cytokines in periaortic adipose tissue surrounding abdominal aortas and increases AngII-induced AAAs.
Methods and results: Hypertrophied white adipocytes surrounded abdominal aortas, whereas brown adipocytes surrounded thoracic aortas of obese mice. mRNA abundance of macrophage proinflammatory chemokines and their receptors were elevated with obesity to a greater extent in abdominal compared to thoracic periaortic adipose tissue. Periaortic adipose tissue explants surrounding abdominal aortas of obese mice released greater concentrations of MCP-1 and promoted more macrophage migration than explants from thoracic aortas. Male C57BL/6 mice were fed a high-fat (HF) diet for 1, 2, or 4 months and then infused with AngII (1000 ng/kg/min) for 28 days. AAA incidence increased progressively with the duration of HF feeding (18%, 36%,and 60%, respectively). Similarly, AngII-infused ob/ob mice exhibited increased AAAs compared to lean controls (76% compared to 32%, respectively, P<0.05). Infusion of AngII to obese mice promoted further macrophage infiltration into periaortic and visceral adipose tissue, and obese mice exhibiting AAAs had greater macrophage content in visceral adipose tissue than mice not developing AAAs.
Conclusions: Increased macrophage accumulation in periaortic adipose tissue surrounding abdominal aortas of AngII-infused obese mice is associated with enhanced AAA formation.
Figures

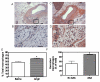
 m) from ob/+ (B,D) and ob/ob (C,E) mice were stained with hematoxylin and eosin (H&E). Adipose tissue surrounding thoracic aortas was composed of multilocular brown adipocytes (B: 40X; insets: 10X). In contrast, adipocytes surrounding abdominal aortas were primarily unilocular (C: 40X; insets: 10X). With obesity (C,F), both brown and white adipocytes surrounding aortas were hypertrophied.
m) from ob/+ (B,D) and ob/ob (C,E) mice were stained with hematoxylin and eosin (H&E). Adipose tissue surrounding thoracic aortas was composed of multilocular brown adipocytes (B: 40X; insets: 10X). In contrast, adipocytes surrounding abdominal aortas were primarily unilocular (C: 40X; insets: 10X). With obesity (C,F), both brown and white adipocytes surrounding aortas were hypertrophied.

 , significantly different from HF-thoracic. Data are mean ± SEM from n = 5 mice/group.
, significantly different from HF-thoracic. Data are mean ± SEM from n = 5 mice/group.
References
-
- Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990;10:497–511. - PubMed
-
- Nieves DJ, Cnop M, Retzlaff B, Walden CE, Brunzell JD, Knopp RH, Kahn SE. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes. 2003;52:172–179. - PubMed
-
- Barandier C, Montani JP, Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol. 2005;289:H1807–13. - PubMed
-
- Eringa EC, Bakker W, Smulders YM, Serne EH, Yudkin JS, Stehouwer CD. Regulation of vascular function and insulin sensitivity by adipose tissue: focus on perivascular adipose tissue. Microcirculation. 2007;14:389–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

