3D timelapse analysis of muscle satellite cell motility
- PMID: 19609936
- PMCID: PMC2798070
- DOI: 10.1002/stem.178
3D timelapse analysis of muscle satellite cell motility
Abstract
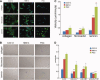
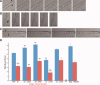
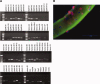
Skeletal muscle repair and regeneration requires the activity of satellite cells, a population of myogenic stem cells scattered throughout the tissue and activated to proliferate and differentiate in response to myotrauma or disease. While it seems likely that satellite cells would need to navigate local muscle tissue to reach damaged areas, relatively little data on such motility exist, and most studies have been with immortalized cell lines. We find that primary satellite cells are significantly more motile than myoblast cell lines, and that adhesion to laminin promotes primary cell motility more than fourfold over other substrates. Using timelapse videomicroscopy to assess satellite cell motility on single living myofibers, we have identified a requirement for the laminin-binding integrin alpha 7 beta 1 in satellite cell motility, as well as a role for hepatocyte growth factor in promoting directional persistence. The extensive migratory behavior of satellite cells resident on muscle fibers suggests caution when determining, based on fixed specimens, whether adjacent cells are daughters from the same mother cell. We also observed more persistent long-term contact between individual satellite cells than has been previously supposed, potential cell-cell attractive and repulsive interactions, and migration between host myofibers. Based on such activity, we assayed for expression of "pathfinding" cues, and found that satellite cells express multiple guidance ligands and receptors. Together, these data suggest that satellite cell migration in vivo may be more extensive than currently thought, and could be regulated by combinations of signals, including adhesive haptotaxis, soluble factors, and guidance cues.
Figures




References
-
- Hawke TJ, Garry DJ. Myogenic satellite cells: Physiology to molecular biology. J Appl Physiol. 2001;91:534–551. - PubMed
-
- Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006;20:1692–1708. - PubMed
-
- Partridge TA, Morgan JE, Coulton GR, et al. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. - PubMed
-
- Péault B, Rudnicki M, Torrente Y, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–877. - PubMed
-
- Skuk D, Goulet M, Roy B, et al. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J Neuropathol Exp Neurol. 2006;65:371–386. - PubMed

