Caveolin-1 promotes resistance to chemotherapy-induced apoptosis in Ewing's sarcoma cells by modulating PKCalpha phosphorylation
- PMID: 19609943
- PMCID: PMC2794946
- DOI: 10.1002/ijc.24754
Caveolin-1 promotes resistance to chemotherapy-induced apoptosis in Ewing's sarcoma cells by modulating PKCalpha phosphorylation
Abstract
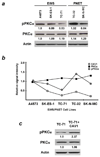
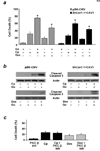
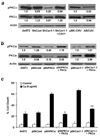
Caveolin-1 (CAV1) has been implicated in the regulation of several signaling pathways and in oncogenesis. Previously, we identified CAV1 as a key determinant of the oncogenic phenotype and tumorigenic activity of cells from tumors of the Ewing's Sarcoma Family (ESFT). However, the possible CAV1 involvement in the chemotherapy resistance commonly presented by an ESFT subset has not been established to date. This report shows that CAV1 expression determines the sensitivity of ESFT cells to clinically relevant chemotherapeutic agents. Analyses of endogenous CAV1 levels in several ESFT cells and ectopic CAV1 expression into ESFT cells expressing low endogenous CAV1 showed that the higher the CAV1 levels, the greater their resistance to drug treatment. Moreover, results from antisense- and shRNA-mediated gene expression knockdown and protein re-expression experiments demonstrated that CAV1 increases the resistance of ESFT cells to doxorubicin (Dox)- and cisplatin (Cp)-induced apoptosis by a mechanism involving the activating phosphorylation of PKCalpha. CAV1 knockdown in ESFT cells led to decreased phospho(Thr(638))-PKCalpha levels and a concomitant sensitization to apoptosis, which were reversed by CAV1 re-expression. These results were recapitulated by PKCalpha knockdown and re-expression in ESFT cells in which CAV1 was previously knocked down, thus demonstrating that phospho(Thr(638))-PKCalpha acts downstream of CAV1 to determine the sensitivity of ESFT cells to chemotherapeutic drugs. These data, along with the finding that CAV1 and phospho(Thr(638))-PKCalpha are co-expressed in approximately 45% of ESFT specimens tested, imply that targeting CAV1 and/or PKCalpha may allow the development of new molecular therapeutic strategies to improve the treatment outcome for patients with ESFT.
Figures



References
-
- Rodriguez-Galindo C, Spunt SL, Pappo AS. Treatment of Ewing sarcoma family of tumors: current status and outlook for the future. Med Pediatr Oncol. 2003;40:276–287. - PubMed
-
- Barker LM, Pendergrass TW, Sanders JE, Hawkins DS. Survival after recurrence of Ewing's sarcoma family of tumors. J Clin Oncol. 2005;23:4354–4362. - PubMed
-
- Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, Kovar H, Joubert I, de Jong P, Rouleau G, Aurias A, Thomas G. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–165. - PubMed
-
- Bernstein M, Kovar H, Paulussen M, Randall RL, Schuck A, Teot LA, Juergens H. Ewing's sarcoma family of tumors: current management. The Oncologist. 2006;11:503–519. - PubMed
-
- Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

