Different astroglia permissivity controls the migration of olfactory bulb interneuron precursors
- PMID: 19610095
- PMCID: PMC3817351
- DOI: 10.1002/glia.20918
Different astroglia permissivity controls the migration of olfactory bulb interneuron precursors
Abstract
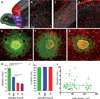
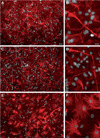
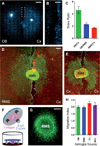
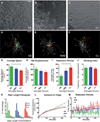
The rostral migratory stream (RMS) is a well defined migratory pathway for precursors of olfactory bulb (OB) interneurons. Throughout the RMS an intense astroglial matrix surrounds the migratory cells. However, it is not clear to what extent the astroglial matrix participates in migration. Here, we have analyzed the migratory behavior of neuroblasts cultured on monolayers of astrocytes isolated from areas that are permissive (RMS and OB) and nonpermissive (cortex and adjacent cortical areas) to migration. Our results demonstrate robust neuroblast migration when RMS-explants are cultured on OB or RMS-astrocytes, in contrast to their behavior on astroglia derived from nonpermissive areas. These differences, mediated by astrocyte-derived nonsoluble factors, are related to the overexpression of extracellular matrix and cell adhesion molecules, as revealed by real-time qRT-PCR. Our results show that astroglia heterogeneity could play a significant role in migration within the RMS and in cell detachment in the OB.
Figures
References
-
- Alves JA, Barone P, Engelender S, Froes MM, Menezes JR. Initial stages of radial glia astrocytic transformation in the early postnatal anterior subventricular zone. J Neurobiol. 2002;52:251–265. - PubMed
-
- Banker G, Goslin K, editors. Culturing nerve cells. Cambridge: MIT Press; 1991. p. 453.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

