Parkin reverses intracellular beta-amyloid accumulation and its negative effects on proteasome function
- PMID: 19610108
- PMCID: PMC2844439
- DOI: 10.1002/jnr.22178
Parkin reverses intracellular beta-amyloid accumulation and its negative effects on proteasome function
Abstract
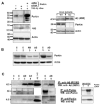
The significance of intracellular beta-amyloid (Abeta(42)) accumulation is increasingly recognized in Alzheimer's disease (AD) pathogenesis. Abeta removal mechanisms that have attracted attention include IDE/neprilysin degradation and antibody-mediated uptake by immune cells. However, the role of the ubiquitin-proteasome system (UPS) in the disposal of cellular Abeta has not been fully explored. The E3 ubiquitin ligase Parkin targets several proteins for UPS degradation, and Parkin mutations are the major cause of autosomal recessive Parkinson's disease. We tested whether Parkin has cross-function to target misfolded proteins in AD for proteasome-dependent clearance in SH-SY5Y and primary neuronal cells. Wild-type Parkin greatly decreased steady-state levels of intracellular Abeta(42), an action abrogated by proteasome inhibitors. Intracellular Abeta(42) accumulation decreased cell viability and proteasome activity. Accordingly, Parkin reversed both effects. Changes in mitochondrial ATP production from Abeta or Parkin did not account for their effects on the proteasome. Parkin knock-down led to accumulation of Abeta. In AD brain, Parkin was found to interact with Abeta and its levels were reduced. Thus, Parkin is cytoprotective, partially by increasing the removal of cellular Abeta through a proteasome-dependent pathway.
Figures



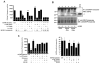
Similar articles
-
Parkin promotes intracellular Abeta1-42 clearance.Hum Mol Genet. 2009 Sep 1;18(17):3206-16. doi: 10.1093/hmg/ddp258. Epub 2009 May 29. Hum Mol Genet. 2009. PMID: 19483198 Free PMC article.
-
Diminished parkin solubility and co-localization with intraneuronal amyloid-β are associated with autophagic defects in Alzheimer's disease.J Alzheimers Dis. 2013;33(1):231-47. doi: 10.3233/JAD-2012-121141. J Alzheimers Dis. 2013. PMID: 22954671
-
Parkin mediates beclin-dependent autophagic clearance of defective mitochondria and ubiquitinated Abeta in AD models.Hum Mol Genet. 2011 Jun 1;20(11):2091-102. doi: 10.1093/hmg/ddr091. Epub 2011 Mar 4. Hum Mol Genet. 2011. PMID: 21378096 Free PMC article.
-
Cross-functional E3 ligases Parkin and C-terminus Hsp70-interacting protein in neurodegenerative disorders.J Neurochem. 2012 Feb;120(3):350-70. doi: 10.1111/j.1471-4159.2011.07588.x. Epub 2011 Dec 21. J Neurochem. 2012. PMID: 22098618 Review.
-
Relationship between amyloid-beta and the ubiquitin-proteasome system in Alzheimer's disease.Neurol Res. 2014 Mar;36(3):276-82. doi: 10.1179/1743132813Y.0000000288. Neurol Res. 2014. PMID: 24512022 Review.
Cited by
-
Parkin prevents cortical atrophy and Aβ-induced alterations of brain metabolism: ¹³C NMR and magnetic resonance imaging studies in AD models.Neuroscience. 2012 Dec 6;225:22-34. doi: 10.1016/j.neuroscience.2012.08.057. Epub 2012 Sep 6. Neuroscience. 2012. PMID: 22960314 Free PMC article.
-
A study of residual lesions in horses that recovered from clinical signs of chronic equine dysautonomia.J Vet Intern Med. 2019 Sep;33(5):2302-2311. doi: 10.1111/jvim.15567. Epub 2019 Jul 22. J Vet Intern Med. 2019. PMID: 31332854 Free PMC article.
-
SQSTM1/p62: A Potential Target for Neurodegenerative Disease.ACS Chem Neurosci. 2019 May 15;10(5):2094-2114. doi: 10.1021/acschemneuro.8b00516. Epub 2019 Apr 19. ACS Chem Neurosci. 2019. PMID: 30657305 Free PMC article. Review.
-
Dysregulation of Ubiquitin-Proteasome System in Neurodegenerative Diseases.Front Aging Neurosci. 2016 Dec 15;8:303. doi: 10.3389/fnagi.2016.00303. eCollection 2016. Front Aging Neurosci. 2016. PMID: 28018215 Free PMC article. Review.
-
Anti-Aβ antibodies bound to neuritic plaques enhance microglia activity and mitigate tau pathology.Acta Neuropathol Commun. 2020 Nov 23;8(1):198. doi: 10.1186/s40478-020-01069-3. Acta Neuropathol Commun. 2020. PMID: 33225991 Free PMC article.
References
-
- Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19:2040–2041. - PubMed
-
- Chafekar SM, Zwart R, Veerhuis R, Vanderstichele H, Baas F, Scheper W. Increased Abeta1–42 production sensitizes neuroblastoma cells for ER stress toxicity. Curr Alzheimer Res. 2008;5:469–474. - PubMed
-
- Chung KK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, Ross CA, Dawson VL, Dawson TM. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med. 2001;7:1144–1150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

