Postnatal development of NT3 and TrkC in mouse ventral cochlear nucleus
- PMID: 19610111
- PMCID: PMC2919343
- DOI: 10.1002/jnr.22179
Postnatal development of NT3 and TrkC in mouse ventral cochlear nucleus
Abstract
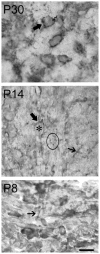
In the developing nervous system, neurotrophin 3 (NT3) and brain-derived neurotrophic factor (BDNF) have been shown to interact with each other and with different parts of a neuron or glia and over considerable distances in time and space. The auditory system provides a useful model for analyzing these events, insofar as it is subdivided into well-defined groups of specific neuronal types that are readily related to each other at each stage of development. Previous work in our laboratory suggested that NT3 and its receptor TrkC in the mouse cochlear nucleus (CN) may be involved in directing neuronal migration and initial targeting of inputs from cochlear nerve axons in the embryo. NT3 is hard to detect soon after birth, but TrkC lingers longer. Here we found NT3 and TrkC around P8 and the peak around P30. Prominent in ventral CN, associated with globular bushy cells and stellate cells, they were localized to different subcellular sites. The TrkC immunostain was cytoplasmic, and that of NT3 was axonal and perisomatic. TrkC may be made by CN neurons, whereas NT3 has a cochlear origin. The temporal pattern of their development and the likelihood of activity-dependent release of NT3 from cochlear axons suggest that it may not be critical in early synaptogenesis; it may provide long-term trophic effects, including stabilization of synapses once established. Activity-related regulation could coordinate the supply of NT3 with inner ear activity. This may require interaction with other neurotrophins, such as BDNF.
Figures





References
-
- Agerman K, Hjerling-Leffler J, Blanchard MP, Scarfone E, Canlon B, Nosrat C, Ernfors P. BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system. Development. 2003;130:1479–1491. - PubMed
-
- Althaus HH, Richter-Landberg C. Glial cells as targets and producers of neurotrophins. Int Rev Cytol. 2000;197:203–277. - PubMed
-
- Bianchi LM, Conover JC, Fritzsch B, DeChiara T, Lindsay RM, Yancopoulos GD. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and honoaygous BDNF null mutant mice. Development. 1996;122:1965–1973. - PubMed
-
- Brumwell CL, Hossain WA, Morest DK, Bernd P. Role for basic fibroblast growth factor (FGF-2) and TrkB expression in the development of the inner ear: in vitro and in situ studies. Exp Neurol. 1999;162:121–145. - PubMed
-
- Brumwell CL, Hossain WA, Morest DK, Wolf B. Biotinidase reveals the morphogenetic sequence in cochlea and cochlear nucleus of mice. Hear Res. 2005;209:104–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

