Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic targets and adduct formation
- PMID: 19610654
- PMCID: PMC4452948
- DOI: 10.1021/tx900147g
Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic targets and adduct formation
Abstract
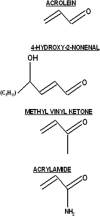
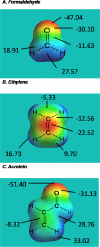
Acrolein and 4-hydroxy-2-nonenal (HNE) are byproducts of lipid peroxidation and are thought to play central roles in various traumatic injuries and disease states that involve cellular oxidative stress, for example, spinal cord trauma, diabetes, and Alzheimer's disease. In this review, we will discuss the chemical attributes of acrolein and HNE that determine their toxicities. Specifically, these aldehydes are classified as type 2 alkenes and are characterized by an alpha,beta-unsaturated carbonyl structure. This structure is a conjugated system that contains mobile pi-electrons. The carbonyl oxygen atom is electronegative and can promote the withdrawal of mobile electron density from the beta-carbon atom causing regional electron deficiency. On the basis of this type of electron polarizability, both acrolein and HNE are considered to be soft electrophiles that preferentially form 1,4-Michael type adducts with soft nucleophiles. Proteomic, quantum mechanical, and kinetic data will be presented, indicating that cysteine sulfhydryl groups are the primary soft nucleophilic targets of acrolein and HNE. This is in contrast to nitrogen groups on harder biological nucleophiles such as lysine or histidine residues. The toxicological outcome of adduct formation is not only dependent upon residue selectivity but also the importance of the targeted amino acid in protein function or structure. In attempting to discern the toxicological significance of a given adduct, we will consider the normal roles of cysteine, lysine, and histidine residues in proteins and the relative merits of corresponding adducts in the manifestations of diseases or toxic states. Understanding the molecular actions of acrolein and HNE could provide insight into many pathogenic conditions that involve initial cellular oxidative stress and could, thereby, offer new efficacious avenues of pharmacological defense.
Figures
Similar articles
-
Molecular mechanisms of the conjugated alpha,beta-unsaturated carbonyl derivatives: relevance to neurotoxicity and neurodegenerative diseases.Toxicol Sci. 2008 Aug;104(2):235-49. doi: 10.1093/toxsci/kfm301. Epub 2007 Dec 13. Toxicol Sci. 2008. PMID: 18083715 Free PMC article. Review.
-
Natural polyphenols as direct trapping agents of lipid peroxidation-derived acrolein and 4-hydroxy-trans-2-nonenal.Chem Res Toxicol. 2009 Oct;22(10):1721-7. doi: 10.1021/tx900221s. Chem Res Toxicol. 2009. PMID: 19743801
-
Synaptosomal toxicity and nucleophilic targets of 4-hydroxy-2-nonenal.Toxicol Sci. 2009 Jan;107(1):171-81. doi: 10.1093/toxsci/kfn226. Epub 2008 Nov 7. Toxicol Sci. 2009. PMID: 18996889 Free PMC article.
-
Reactions of electrophiles with nucleophilic thiolate sites: relevance to pathophysiological mechanisms and remediation.Free Radic Res. 2016;50(2):195-205. doi: 10.3109/10715762.2015.1094184. Epub 2015 Nov 11. Free Radic Res. 2016. PMID: 26559119 Free PMC article. Review.
-
Structure-toxicity analysis of type-2 alkenes: in vitro neurotoxicity.Toxicol Sci. 2007 Jan;95(1):136-46. doi: 10.1093/toxsci/kfl127. Epub 2006 Oct 5. Toxicol Sci. 2007. PMID: 17023561
Cited by
-
Role of aldose reductase in the metabolism and detoxification of carnosine-acrolein conjugates.J Biol Chem. 2013 Sep 27;288(39):28163-79. doi: 10.1074/jbc.M113.504753. Epub 2013 Aug 8. J Biol Chem. 2013. PMID: 23928303 Free PMC article.
-
Oxidized lipids activate autophagy in a JNK-dependent manner by stimulating the endoplasmic reticulum stress response.Redox Biol. 2013 Jan 26;1(1):56-64. doi: 10.1016/j.redox.2012.10.003. eCollection 2013. Redox Biol. 2013. PMID: 24024137 Free PMC article.
-
Modulation of keratinocyte expression of antioxidants by 4-hydroxynonenal, a lipid peroxidation end product.Toxicol Appl Pharmacol. 2014 Mar 1;275(2):113-21. doi: 10.1016/j.taap.2014.01.001. Epub 2014 Jan 11. Toxicol Appl Pharmacol. 2014. PMID: 24423726 Free PMC article.
-
The tobacco smoke component acrolein induces glucocorticoid resistant gene expression via inhibition of histone deacetylase.Toxicol Lett. 2016 Jan 5;240(1):43-9. doi: 10.1016/j.toxlet.2015.10.009. Epub 2015 Oct 19. Toxicol Lett. 2016. PMID: 26481333 Free PMC article.
-
Lipoxin A4 yields an electrophilic 15-oxo metabolite that mediates FPR2 receptor-independent anti-inflammatory signaling.bioRxiv [Preprint]. 2024 Feb 7:2024.02.06.579101. doi: 10.1101/2024.02.06.579101. bioRxiv. 2024. Update in: J Lipid Res. 2025 Jan;66(1):100705. doi: 10.1016/j.jlr.2024.100705. PMID: 38370667 Free PMC article. Updated. Preprint.
References
-
- Esterbauer H, Schaur RJ, Zollner J. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Rad. Biol. Med. 1991;11:81–128. - PubMed
-
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J. Neurochem. 2006;97:1634–1658. - PubMed
-
- Lefer DJ, Granger N. Oxidative stress and cardiac disease. Am. J. Med. 2000;109:316–323. - PubMed
-
- Lieber CS. Alcoholic liver injury: pathogenesis and therapy in 2001. Pathol. Biol. 2001;49:738–752. - PubMed
-
- Povlishock JT, Kontos HA. The role of oxygen radicals in the pathobiology of traumatic brain injury. Hum Cell. 1992;5:345–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

