Antiphospholipid antibodies induce a pro-inflammatory response in first trimester trophoblast via the TLR4/MyD88 pathway
- PMID: 19614626
- PMCID: PMC2772057
- DOI: 10.1111/j.1600-0897.2009.00717.x
Antiphospholipid antibodies induce a pro-inflammatory response in first trimester trophoblast via the TLR4/MyD88 pathway
Abstract
Problem: Women with antiphospholipid antibodies (aPL) are at risk for recurrent miscarriage, pre-eclampsia, and pre-term labor. aPL target the placenta directly by binding to beta(2)-glycoprotein I (beta(2)GPI) expressed on the surface of trophoblast cells. The objective of this study was to determine the effects of aPL on trophoblast function and the mechanisms involved.
Method of study: First trimester trophoblast cells were treated with anti-beta(2)GPI monoclonal antibodies and patient-derived aPL, after which cell survival and function was evaluated.
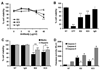
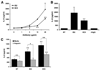
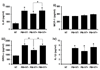
Results: We report that anti-beta(2)GPI antibodies trigger an inflammatory response in trophoblast, characterized by increased secretion of interleukin (IL)-8, MCP-1, GRO-alpha, and IL-1beta, and that this occurs in a TLR-4/MyD88-dependent manner. At high concentrations, these antibodies also induce caspase-mediated cell death. This was attenuated upon disabling of the MyD88 pathway, suggesting that anti-beta(2)GPI-induced inflammatory mediators compromise trophoblast survival by acting in an autocrine/paracrine manner. Enhanced IL-8, GRO-alpha, and IL-1beta secretion also occurred when trophoblast cells were incubated with antibodies from patients with antiphospholipid syndrome. Heparin, which acts as a pro-survival factor in human trophoblast, attenuated the anti-beta(2)GPI antibody-mediated cell death, and also the pro-inflammatory response, but only at high concentrations.
Conclusion: These findings demonstrate that aPL triggers a placental inflammatory response via the TLR-4/MyD88 pathway, which in turn compromises trophoblast survival. Thus, the TLR-4/MyD88 pathway may provide a new therapeutic target to improve pregnancy outcome in antiphospholipid syndrome patients.
Figures

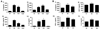



Similar articles
-
Modulation of trophoblast angiogenic factor secretion by antiphospholipid antibodies is not reversed by heparin.Am J Reprod Immunol. 2011 Oct;66(4):286-96. doi: 10.1111/j.1600-0897.2011.01007.x. Epub 2011 May 4. Am J Reprod Immunol. 2011. PMID: 21545366
-
A role for uric acid and the Nalp3 inflammasome in antiphospholipid antibody-induced IL-1β production by human first trimester trophoblast.PLoS One. 2013 Jun 6;8(6):e65237. doi: 10.1371/journal.pone.0065237. Print 2013. PLoS One. 2013. PMID: 23762324 Free PMC article.
-
Pravastatin does not prevent antiphospholipid antibody-mediated changes in human first trimester trophoblast function.Hum Reprod. 2012 Oct;27(10):2933-40. doi: 10.1093/humrep/des288. Epub 2012 Aug 11. Hum Reprod. 2012. PMID: 22888169
-
Pregnancies complicated with antiphospholipid syndrome: the pathogenic mechanism of antiphospholipid antibodies: a review of the literature.Ann N Y Acad Sci. 2007 Jun;1108:505-14. doi: 10.1196/annals.1422.054. Ann N Y Acad Sci. 2007. PMID: 17894016 Review.
-
Updating on the pathogenic mechanisms 5 of the antiphospholipid antibodies-associated pregnancy loss.Clin Rev Allergy Immunol. 2008 Jun;34(3):332-7. doi: 10.1007/s12016-007-8055-9. Clin Rev Allergy Immunol. 2008. PMID: 18175073 Review.
Cited by
-
Nod1, but not the ASC inflammasome, contributes to induction of IL-1β secretion in human trophoblasts after sensing of Chlamydia trachomatis.Mucosal Immunol. 2013 Mar;6(2):235-43. doi: 10.1038/mi.2012.63. Epub 2012 Jul 4. Mucosal Immunol. 2013. PMID: 22763410 Free PMC article.
-
Obstetric Anti-phospholipid Syndrome: State of the Art.Curr Rheumatol Rep. 2018 Aug 13;20(10):59. doi: 10.1007/s11926-018-0772-y. Curr Rheumatol Rep. 2018. PMID: 30105597 Review.
-
Effects of anti-beta 2-glycoprotein 1 antibodies and its association with pregnancy-related morbidity in antiphospholipid syndrome.Am J Reprod Immunol. 2022 Jan;87(1):e13509. doi: 10.1111/aji.13509. Epub 2021 Dec 4. Am J Reprod Immunol. 2022. PMID: 34738282 Free PMC article. Review.
-
The Weight of IgA Anti-β2glycoprotein I in the Antiphospholipid Syndrome Pathogenesis: Closing the Gap of Seronegative Antiphospholipid Syndrome.Int J Mol Sci. 2020 Nov 26;21(23):8972. doi: 10.3390/ijms21238972. Int J Mol Sci. 2020. PMID: 33255963 Free PMC article. Review.
-
DAMPening inflammation by modulating TLR signalling.Mediators Inflamm. 2010;2010:672395. doi: 10.1155/2010/672395. Epub 2010 Jul 13. Mediators Inflamm. 2010. PMID: 20706656 Free PMC article. Review.
References
-
- D'Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007;369:587–596. - PubMed
-
- Valesini G, Alessandri C. New facet of antiphospholipid antibodies. Ann N Y Acad Sci. 2005;1051:487–497. - PubMed
-
- Rai RS, Regan L, Clifford K, Pickering W, Dave M, Mackie I, McNally T, Cohen H. Antiphospholipid antibodies and beta 2-glycoprotein-I in 500 women with recurrent miscarriage: results of a comprehensive screening approach. Hum Reprod. 1995;10:2001–2005. - PubMed
-
- Regan L, Rai R. Epidemiology and the medical causes of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:839–854. - PubMed
-
- Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, Jacobsen S, Lakos G, Tincani A, Kontopoulou-Griva I, Galeazzi M, Meroni PL, Derksen RH, de Groot PG, Gromnica-Ihle E, Baleva M, Mosca M, Bombardieri S, Houssiau F, Gris JC, Quere I, Hachulla E, Vasconcelos C, Roch B, Fernandez-Nebro A, Boffa MC, Hughes GR, Ingelmo M. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019–1027. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

