Results of treatment with telmisartan-amlodipine in hypertensive patients
- PMID: 19614805
- PMCID: PMC8673089
- DOI: 10.1111/j.1751-7176.2009.00098.x
Results of treatment with telmisartan-amlodipine in hypertensive patients
Abstract
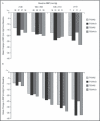
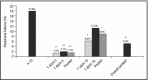
This randomized 4 x 4 factorial study determined the efficacy and safety of telmisartan (T) plus amlodipine (A) in hypertensive patients. Adults (N=1461) with stage 1 or 2 hypertension (baseline blood pressure [BP]: 153.2[12.1]/101.7[4.3] mm Hg) were randomized to 1 of 16 treatment groups with T 0, 20, 40, 80 mg and A 0, 2.5, 5, 10 mg for 8 weeks. In-clinic BP reductions were greater with combination therapy than respective monotherapies. The greatest least-square mean systolic/diastolic BP reductions were observed with T80 mg plus A10 mg (-26.4/-20.1 mm Hg; P<.05 compared with both monotherapies). BP control was also greatest in the T80-mg plus A10-mg group (76.5% [overall control] and 85.3% [diastolic BP control]), and BP response rates >90% with this combination. Peripheral edema was most common in the A10-mg group (17.8%); however, this rate was notably lower when A was used in combination with T: 11.4% (T20/A10), 6.2% (T40/A10), and 11.3% (T80/A10).
Figures

References
-
- Wang YR, Alexander GC, Stafford RS. Outpatient hypertension treatment, treatment intensification, and control in Western Europe and the United States. Arch Intern Med. 2007;167:141–147. - PubMed
-
- Weir MR. Targeting mechanisms of hypertensive vascular disease with dual calcium channel and renin‐angiotensin system blockade. J Hum Hypertens. 2007;21:770–779. - PubMed
-
- Epstein M, Bakris G. Newer approaches to antihypertensive therapy. Use of fixed‐dose combination therapy. Arch Intern Med. 1996;156:1969–1978. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

