Activation of latent transforming growth factor-beta1 by nitric oxide in macrophages: role of soluble guanylate cyclase and MAP kinases
- PMID: 19614923
- PMCID: PMC3654795
- DOI: 10.1111/j.1524-475X.2009.00509.x
Activation of latent transforming growth factor-beta1 by nitric oxide in macrophages: role of soluble guanylate cyclase and MAP kinases
Abstract
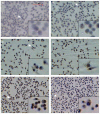
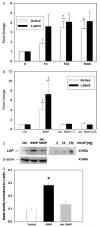
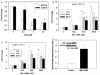
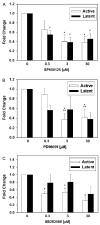
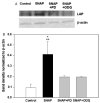
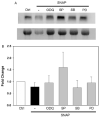
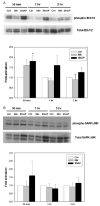
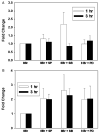
The inducible nitric oxide (NO) synthase and the cytokine transforming growth factor-beta1 (TGF-beta1), both central modulators of wound healing, interact reciprocally: TGF-beta1 generally suppresses iNOS expression, while NO can induce and activate latent TGF-beta1. We have shown that chemical NO activates recombinant human latent TGF-beta1 by S-nitrosation of the latency-associated peptide (LAP), a cleaved portion of pro-TGF-beta1 that maintains TGF-beta1 in a biologically-inactive state. We hypothesized that cell-associated TGF-beta1 could be activated by NO via known NO-inducible signaling pathways (soluble guanylate cyclase [sGC] and mitogen-activated protein [MAP] kinases). Treatment of mouse RAW 264.7 macrophage-like cells with the NO donor S-nitroso-N-acetyl-D,L-penicillamine (SNAP) led to a dose- and time-dependent increase in cell-associated active and latent TGF-beta1, as assessed by quantitative immunocytochemistry for active TGF-beta1 vs. LAP and partially validated by western blot analysis. Treatment with the sGC inhibitor 1,H-[1,2,4]oxadiazole[4,3-a]quinoxalon-1-one (ODQ) reduced both active and latent TGF-beta1 dose-dependently. SNAP, in the presence or absence of ODQ or the MAP kinase inhibitors, did not affect steady-state TGF-beta1 mRNA levels. Treatment with inhibitors specific for JNK1/2, ERK1/2, and p38 MAP kinases suppressed SNAP-induced active and latent TGF-beta1. Treatment with the cell-permeable cGMP analog 8-Br-cGMP increased both active and latent TGF-beta1. However, TGF-beta1 activation induced by 8-Br-cGMP was not blocked by MAP kinase inhibitors. Our findings suggest that NO activates latent TGF-beta1 via activation of sGC and generation of cGMP and separately via MAP kinase activation, and may shed insight into the mechanisms by which both cGMP production and MAP kinase activation enhance wound healing.
Figures
Similar articles
-
In vivo, in vitro, and in silico studies suggest a conserved immune module that regulates malaria parasite transmission from mammals to mosquitoes.J Theor Biol. 2013 Oct 7;334:173-86. doi: 10.1016/j.jtbi.2013.05.028. Epub 2013 Jun 11. J Theor Biol. 2013. PMID: 23764028 Free PMC article.
-
Expression and activity of soluble guanylate cyclase in injury and repair of anti-thy1 glomerulonephritis.Kidney Int. 2004 Dec;66(6):2224-36. doi: 10.1111/j.1523-1755.2004.66012.x. Kidney Int. 2004. PMID: 15569311
-
Regulation of transforming growth factor beta1 by nitric oxide.Cancer Res. 1999 May 1;59(9):2142-9. Cancer Res. 1999. PMID: 10232601
-
Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3',5'-cyclic guanosine monophosphate/protein kinase G signaling pathway: an in vitro study.Endocrinology. 2003 Jul;144(7):3114-29. doi: 10.1210/en.2002-0167. Endocrinology. 2003. PMID: 12810568
-
Photobiomodulation-Activated Latent Transforming Growth Factor-β1: A Critical Clinical Therapeutic Pathway and an Endogenous Optogenetic Tool for Discovery.Photobiomodul Photomed Laser Surg. 2022 Feb;40(2):136-147. doi: 10.1089/photob.2021.0109. Epub 2021 Dec 14. Photobiomodul Photomed Laser Surg. 2022. PMID: 34905400 Review.
Cited by
-
Identification of a novel pathway of transforming growth factor-β1 regulation by extracellular NAD+ in mouse macrophages: in vitro and in silico studies.J Biol Chem. 2012 Sep 7;287(37):31003-14. doi: 10.1074/jbc.M112.344309. Epub 2012 Jul 24. J Biol Chem. 2012. PMID: 22829588 Free PMC article.
-
Evaluating the effect of cold plasma on the healing of gingival wound.J Diabetes Metab Disord. 2021 May 2;20(1):741-745. doi: 10.1007/s40200-021-00810-6. eCollection 2021 Jun. J Diabetes Metab Disord. 2021. PMID: 34222088 Free PMC article.
-
Bitter taste receptors stimulate phagocytosis in human macrophages through calcium, nitric oxide, and cyclic-GMP signaling.Cell Mol Life Sci. 2021 Jan;78(1):271-286. doi: 10.1007/s00018-020-03494-y. Epub 2020 Mar 14. Cell Mol Life Sci. 2021. PMID: 32172302 Free PMC article.
-
The changing role of TGFβ in healthy, ageing and osteoarthritic joints.Nat Rev Rheumatol. 2017 Mar;13(3):155-163. doi: 10.1038/nrrheum.2016.219. Epub 2017 Feb 2. Nat Rev Rheumatol. 2017. PMID: 28148919 Review.
-
Nitric oxide synthase-3 promotes embryonic development of atrioventricular valves.PLoS One. 2013 Oct 29;8(10):e77611. doi: 10.1371/journal.pone.0077611. eCollection 2013. PLoS One. 2013. PMID: 24204893 Free PMC article.
References
-
- Hart J. Inflammation. 1: its role in the healing of acute wounds. J Wound Care. 2002;11:205–9. - PubMed
-
- Schwentker A, Vodovotz Y, Weller R, Billiar TR. Nitric oxide and wound repair: role of cytokines? Nitric Oxide. 2002;7:1–10. - PubMed
-
- Roberts AB, Sporn MB. Transforming growth factor-β. In: Clark RAF, editor. The molecular and cellular biology of wound repair. Plenum Press; New York: 1996. pp. 275–308.
-
- Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280:7409–12. - PubMed
-
- Ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29:265–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

