DNA damage induces nuclear translocation of parkin
- PMID: 19615059
- PMCID: PMC2720942
- DOI: 10.1186/1423-0127-16-67
DNA damage induces nuclear translocation of parkin
Abstract
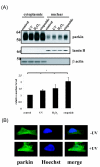
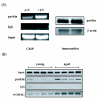
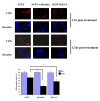
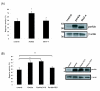
Parkinson's disease (PD) is the second most common form of human degenerative disorder. Mutation of parkin is one of the most prevalent causes of autosomal recessive PD. Parkin is an E3 ubiquitin ligase that acts on a variety of substrates, resulting in polyubiquitination and degradation by the proteasome or monoubiquitination and regulation of biological activity. However, the cellular functions of parkin that relate to its pathological involvement in PD are not well understood. Here I show that parkin translocates into nucleus upon DNA damage. Nuclear translocation of parkin appears to be required to promote DNA repair. These findings suggest that DNA damage induces nuclear translocation of parkin leading to the PCNA interaction and possibly other nuclear proteins involved in DNA repair. These results suggest that parkin promotes DNA repair and protects against genotoxicity, and implicate DNA damage as a potential pathogenic mechanism in parkinsonism.
Figures
Similar articles
-
Regulation of DNA repair by parkin.Biochem Biophys Res Commun. 2009 May 1;382(2):321-5. doi: 10.1016/j.bbrc.2009.03.048. Epub 2009 Mar 13. Biochem Biophys Res Commun. 2009. PMID: 19285961
-
[Etiology and pathogenesis of Parkinson's disease: from mitochondrial dysfunctions to familial Parkinson's disease].Rinsho Shinkeigaku. 2004 Apr-May;44(4-5):241-62. Rinsho Shinkeigaku. 2004. PMID: 15287506 Review. Japanese.
-
Dyrk1A phosphorylates parkin at Ser-131 and negatively regulates its ubiquitin E3 ligase activity.J Neurochem. 2015 Aug;134(4):756-68. doi: 10.1111/jnc.13164. Epub 2015 Jun 3. J Neurochem. 2015. PMID: 25963095
-
Parkin-mediated monoubiquitination of the PDZ protein PICK1 regulates the activity of acid-sensing ion channels.Mol Biol Cell. 2007 Aug;18(8):3105-18. doi: 10.1091/mbc.e05-11-1027. Epub 2007 Jun 6. Mol Biol Cell. 2007. PMID: 17553932 Free PMC article.
-
Linking F-box protein 7 and parkin to neuronal degeneration in Parkinson's disease (PD).Mol Brain. 2016 Apr 18;9:41. doi: 10.1186/s13041-016-0218-2. Mol Brain. 2016. PMID: 27090516 Free PMC article. Review.
Cited by
-
Mitochondrial dysfunction in Parkinson's disease.Parkinsons Dis. 2011 Mar 15;2011:716871. doi: 10.4061/2011/716871. Parkinsons Dis. 2011. PMID: 21461368 Free PMC article.
-
Parkin, A Top Level Manager in the Cell's Sanitation Department.Open Biochem J. 2011;5:9-26. doi: 10.2174/1874091X01105010009. Epub 2011 Apr 18. Open Biochem J. 2011. PMID: 21633666 Free PMC article.
-
RBR E3 ubiquitin ligases: new structures, new insights, new questions.Biochem J. 2014 Mar 15;458(3):421-37. doi: 10.1042/BJ20140006. Biochem J. 2014. PMID: 24576094 Free PMC article. Review.
-
Activated or Impaired: An Overview of DNA Repair in Neurodegenerative Diseases.Aging Dis. 2022 Jul 11;13(4):987-1004. doi: 10.14336/AD.2021.1212. eCollection 2022 Jul 11. Aging Dis. 2022. PMID: 35855336 Free PMC article.
-
Tumor suppressor Parkin induces p53-mediated cell cycle arrest in human lung and colorectal cancer cells.BMB Rep. 2023 Oct;56(10):557-562. doi: 10.5483/BMBRep.2023-0134. BMB Rep. 2023. PMID: 37679297 Free PMC article.
References
-
- Hedrich K, Eskelson C, Wilmot B, Marder K, Harris J, Garrels J, Meija-Santana H, Vieregge P, Jacobs H, Bressman SB, Lang AE, Kann M, Abbruzzese G, Martinelli P, Schwinger E, Ozelius LJ, Pramstaller PP, Klein C, Kramer P. Distribution, type, and origin of Parkin mutations: review and case studies. Mov Disord. 2004;19(10):1146–1157. doi: 10.1002/mds.20234. - DOI - PubMed
-
- Cesari R, Martin ES, Calin GA, Pentimalli F, Bichi R, McAdams H, Trapasso F, Drusco A, Shimizu M, Masciullo V, d'Andrilli G, Scambia G, Picchio MC, Alder H, Godwin AK, Croce CM. Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25-q27. Proc Nat Acad Sci. 2003;100(10):5956–5961. doi: 10.1073/pnas.0931262100. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

