Modulation of lung inflammation by vessel dilator in a mouse model of allergic asthma
- PMID: 19615076
- PMCID: PMC2716304
- DOI: 10.1186/1465-9921-10-66
Modulation of lung inflammation by vessel dilator in a mouse model of allergic asthma
Abstract
Background: Atrial natriuretic peptide (ANP) and its receptor, NPRA, have been extensively studied in terms of cardiovascular effects. We have found that the ANP-NPRA signaling pathway is also involved in airway allergic inflammation and asthma. ANP, a C-terminal peptide (amino acid 99-126) of pro-atrial natriuretic factor (proANF) and a recombinant peptide, NP73-102 (amino acid 73-102 of proANF) have been reported to induce bronchoprotective effects in a mouse model of allergic asthma. In this report, we evaluated the effects of vessel dilator (VD), another N-terminal natriuretic peptide covering amino acids 31-67 of proANF, on acute lung inflammation in a mouse model of allergic asthma.
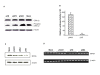
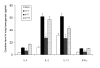
Methods: A549 cells were transfected with pVD or the pVAX1 control plasmid and cells were collected 24 hrs after transfection to analyze the effect of VD on inactivation of the extracellular-signal regulated receptor kinase (ERK1/2) through western blot. Luciferase assay, western blot and RT-PCR were also performed to analyze the effect of VD on NPRA expression. For determination of VD's attenuation of lung inflammation, BALB/c mice were sensitized and challenged with ovalbumin and then treated intranasally with chitosan nanoparticles containing pVD. Parameters of airway inflammation, such as airway hyperreactivity, proinflammatory cytokine levels, eosinophil recruitment and lung histopathology were compared with control mice receiving nanoparticles containing pVAX1 control plasmid.
Results: pVD nanoparticles inactivated ERK1/2 and downregulated NPRA expression in vitro, and intranasal treatment with pVD nanoparticles protected mice from airway inflammation.
Conclusion: VD's modulation of airway inflammation may result from its inactivation of ERK1/2 and downregulation of NPRA expression. Chitosan nanoparticles containing pVD may be therapeutically effective in preventing allergic airway inflammation.
Figures

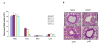
References
-
- Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116(2):377–383. - PubMed
-
- Holgate ST, Davies DE, Lackie PM, Wilson SJ, Puddicombe SM, Lordan JL. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol. 2000;105(2 Pt 1):193–204. - PubMed
-
- Agarwal SK, Marshall GD., Jr Dexamethasone promotes type 2 cytokine production primarily through inhibition of type 1 cytokines. J Interferon Cytokine Res. 2001;21(3):147–155. - PubMed
-
- Huang MT, Yang YH, Lin YT, Lu MY, Wang LH, Tsai MJ, Chiang BL. Beta2-agonist exerts differential effects on the development of cord blood T cells but not on peripheral blood T cells. Pediatr Allergy Immunol. 2001;12(1):17–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

