Mechanical strain induces involution-associated events in mammary epithelial cells
- PMID: 19615079
- PMCID: PMC2721828
- DOI: 10.1186/1471-2121-10-55
Mechanical strain induces involution-associated events in mammary epithelial cells
Abstract
Background: Shortly after weaning, a complex multi-step process that leads to massive epithelial apoptosis is triggered by tissue local factors in the mouse mammary gland. Several reports have demonstrated the relevance of mechanical stress to induce adaptive responses in different cell types. Interestingly, these signaling pathways also participate in mammary gland involution. Then, it has been suggested that cell stretching caused by milk accumulation after weaning might be the first stimulus that initiates the complete remodeling of the mammary gland. However, no previous report has demonstrated the impact of mechanical stress on mammary cell physiology. To address this issue, we have designed a new practical device that allowed us to evaluate the effects of radial stretching on mammary epithelial cells in culture.
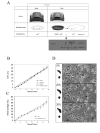
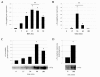
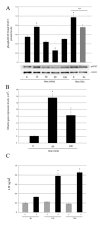
Results: We have designed and built a new device to analyze the biological consequences of applying mechanical stress to cells cultured on flexible silicone membranes. Subsequently, a geometrical model that predicted the percentage of radial strain applied to the elastic substrate was developed. By microscopic image analysis, the adjustment of these calculations to the actual strain exerted on the attached cells was verified. The studies described herein were all performed in the HC11 non-tumorigenic mammary epithelial cell line, which was originated from a pregnant BALB/c mouse. In these cells, as previously observed in other tissue types, mechanical stress induced ERK1/2 phosphorylation and c-Fos mRNA and protein expression. In addition, we found that mammary cell stretching triggered involution associated cellular events as Leukemia Inhibitory Factor (LIF) expression induction, STAT3 activation and AKT phosphorylation inhibition.
Conclusion: Here, we show for the first time, that mechanical strain is able to induce weaning-associated events in cultured mammary epithelial cells. These results were obtained using a new practical and affordable device specifically designed for such a purpose. We believe that our results indicate the relevance of mechanical stress among the early post-lactation events that lead to mammary gland involution.
Figures

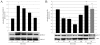
Similar articles
-
Leukemia inhibitory factor induces apoptosis of the mammary epithelial cells and participates in mouse mammary gland involution.Exp Cell Res. 2003 Jan 1;282(1):35-47. doi: 10.1006/excr.2002.5666. Exp Cell Res. 2003. PMID: 12490192
-
Loss of interleukin 6 results in delayed mammary gland involution: a possible role for mitogen-activated protein kinase and not signal transducer and activator of transcription 3.Mol Endocrinol. 2002 Dec;16(12):2902-12. doi: 10.1210/me.2001-0330. Mol Endocrinol. 2002. PMID: 12456808
-
Mouse mammary tumors display Stat3 activation dependent on leukemia inhibitory factor signaling.Breast Cancer Res. 2007;9(5):R69. doi: 10.1186/bcr1777. Breast Cancer Res. 2007. PMID: 17925034 Free PMC article.
-
Molecular mechanism of mammary gland involution: An update.Dev Biol. 2019 Jan 15;445(2):145-155. doi: 10.1016/j.ydbio.2018.11.002. Epub 2018 Nov 15. Dev Biol. 2019. PMID: 30448440 Review.
-
Transcription factor activities and gene expression during mouse mammary gland involution.J Mammary Gland Biol Neoplasia. 1999 Apr;4(2):145-52. doi: 10.1023/a:1018721107061. J Mammary Gland Biol Neoplasia. 1999. PMID: 10426393 Review.
Cited by
-
Rac1 controls cell turnover and reversibility of the involution process in postpartum mammary glands.PLoS Biol. 2023 Jan 19;21(1):e3001583. doi: 10.1371/journal.pbio.3001583. eCollection 2023 Jan. PLoS Biol. 2023. PMID: 36656812 Free PMC article.
-
Design and construction of an equibiaxial cell stretching system that is improved for biochemical analysis.PLoS One. 2014 Mar 13;9(3):e90665. doi: 10.1371/journal.pone.0090665. eCollection 2014. PLoS One. 2014. PMID: 24626190 Free PMC article.
-
PMCA2 regulates apoptosis during mammary gland involution and predicts outcome in breast cancer.Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11405-10. doi: 10.1073/pnas.0911186107. Epub 2010 Jun 4. Proc Natl Acad Sci U S A. 2010. PMID: 20534448 Free PMC article.
-
Collagen and PAPP-A in the Etiology of Postpartum Breast Cancer.Horm Cancer. 2019 Dec;10(4-6):137-144. doi: 10.1007/s12672-019-00368-z. Epub 2019 Oct 20. Horm Cancer. 2019. PMID: 31631239 Free PMC article. Review.
-
Mechanical signaling in reproductive tissues: mechanisms and importance.Reprod Sci. 2014 Sep;21(9):1093-107. doi: 10.1177/1933719114542023. Epub 2014 Jul 6. Reprod Sci. 2014. PMID: 25001021 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

