Homozygosity mapping reveals PDE6C mutations in patients with early-onset cone photoreceptor disorders
- PMID: 19615668
- PMCID: PMC2725240
- DOI: 10.1016/j.ajhg.2009.06.016
Homozygosity mapping reveals PDE6C mutations in patients with early-onset cone photoreceptor disorders
Abstract
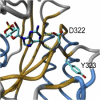
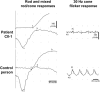
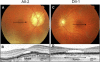
Cone photoreceptor disorders form a clinical spectrum of diseases that include progressive cone dystrophy (CD) and complete and incomplete achromatopsia (ACHM). The underlying disease mechanisms of autosomal recessive (ar)CD are largely unknown. Our aim was to identify causative genes for these disorders by genome-wide homozygosity mapping. We investigated 75 ACHM, 97 arCD, and 20 early-onset arCD probands and excluded the involvement of known genes for ACHM and arCD. Subsequently, we performed high-resolution SNP analysis and identified large homozygous regions spanning the PDE6C gene in one sibling pair with early-onset arCD and one sibling pair with incomplete ACHM. The PDE6C gene encodes the cone alpha subunit of cyclic guanosine monophosphate (cGMP) phosphodiesterase, which converts cGMP to 5'-GMP, and thereby plays an essential role in cone phototransduction. Sequence analysis of the coding region of PDE6C revealed homozygous missense mutations (p.R29W, p.Y323N) in both sibling pairs. Sequence analysis of 104 probands with arCD and 10 probands with ACHM revealed compound heterozygous PDE6C mutations in three complete ACHM patients from two families. One patient had a frameshift mutation and a splice defect; the other two had a splice defect and a missense variant (p.M455V). Cross-sectional retinal imaging via optical coherence tomography revealed a more pronounced absence of cone photoreceptors in patients with ACHM compared to patients with early-onset arCD. Our findings identify PDE6C as a gene for cone photoreceptor disorders and show that arCD and ACHM constitute genetically and clinically overlapping phenotypes.
Figures

References
-
- Michaelides M., Hardcastle A.J., Hunt D.M., Moore A.T. Progressive cone and cone-rod dystrophies: Phenotypes and underlying molecular genetic basis. Surv. Ophthalmol. 2006;51:232–258. - PubMed
-
- Kohl S., Varsanyi B., Antunes G.A., Baumann B., Hoyng C.B., Jagle H., Rosenberg T., Kellner U., Lorenz B., Salati R. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur. J. Hum. Genet. 2005;13:302–308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

