A family of bioreducible poly(disulfide amine)s for gene delivery
- PMID: 19615739
- PMCID: PMC2737532
- DOI: 10.1016/j.biomaterials.2009.06.050
A family of bioreducible poly(disulfide amine)s for gene delivery
Abstract
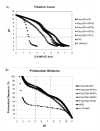
A family of bioreducible poly(disulfide amine)s, which differ in the length of polymethylene spacer [-(CH(2))(n)-] in the main chain and the side chain, has been synthesized. These bioreducible poly(disulfide amine)s exhibit local environment specific degradability and are associated with lower cytotoxicity than branched poly(ethylenimine) (bPEI, 25 kDa). These cationic polymers also show higher buffering capacity and protonation degree than bPEI, facilitating the endosomal escape of carried genetic materials. The transfection efficiency of these agents is oligomethylene length dependent. Poly(cystaminebisacrylamide-spermine) [poly(CBA-SP)], poly(cystaminebisacrylamide-bis(3-aminopropyl)-1,3-propanediamine) [poly(CBA-APPD)], and poly(cyxtaminebisacrylamide-bis(3-aminopropyl)-ethylenediamine) [ploy(CBA-APED)] with longer propylene [-(CH(2))(3)-] side spacer, demonstrate higher transfection efficacy than the counterpart poly(cystaminebisacrylamide-bis(2-aminoethyl)-1,3-propanediamine) [poly(CBA-AEPD)] and poly(cystaminebisacrylamide-triethylenetetramine) [poly(CBA-TETA)], which have shorter ethylene [-(CH(2))(2)-] side spacer. The poly(CBA-SP), poly(CBA-APPD), poly(CBA-APED) with the main chain spacer of -(CH(2))(4)-, -(CH(2))(3)-, -(CH(2))(2)- demonstrate similar transfection efficiency, indicating the length of polymer main chain spacer has less influence on transfection efficiency. However, with the same short ethylene [-(CH(2))(2)-] side spacer, poly(CBA-AEPD), with the longer main chain oligomethylene units [-(CH(2))(3)-], showed relatively higher transfection efficiency than poly(CBA-TETA), having shorter main chain oligomethylene units [-(CH(2))(2)-]. Of these polymeric carriers, poly(CBA-SP) demonstrated the highest transfection in the C2C12 cell line, while poly(CBA-APED) showed the highest transfection in the HeLa cell line. All of these agents showed greater transfection activity than commercialized bPEI 25 kDa. The poly(disulfide amine)s are promising safe and efficient non-viral vectors for gene delivery.
Figures







References
-
- Verma IM, Somia N. Gene therapy -- promises, problems and prospects. Nature. 1997;389(6648):239–242. - PubMed
-
- Lin C, Engbersen JF. Effect of chemical functionalities in poly(amido amine)s for non-viral gene transfection. J Control Release. 2008;132:267–272. - PubMed
-
- Wang XL, Jensen R, Lu ZR. A novel environment-sensitive biodegradable polydisulfide with protonatable pendants for nucleic acid delivery. J Control Release. 2007;120(3):250–258. - PubMed
-
- Li S, Huang L. Nonviral gene therapy: promises and challenges. Gene Ther. 2000;7(1):31–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

